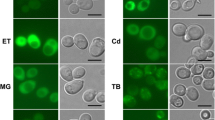
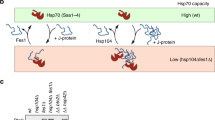
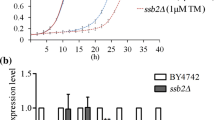
Abstract
As a result of exposure to stress conditions, mutations, or defects during synthesis, cellular proteins are prone to misfold. To cope with such partially denatured proteins, cells mount a regulated transcriptional response involving the Hsf1 transcription factor, which drives the synthesis of molecular chaperones and other stress-relieving proteins. Here, we show that the fission yeast Schizosaccharomyces pombe orthologues of human BAG-1, Bag101, and Bag102, are Hsp70 co-chaperones that associate with 26S proteasomes. Only a subgroup of Hsp70-type chaperones, including Ssa1, Ssa2, and Sks2, binds Bag101 and Bag102 and key residues in the Hsp70 ATPase domains, required for interaction with Bag101 and Bag102, were identified. In humans, BAG-1 overexpression is typically observed in cancers. Overexpression of bag101 and bag102 in fission yeast leads to a strong growth defect caused by triggering Hsp70 to release and activate the Hsf1 transcription factor. Accordingly, the bag101-linked growth defect is alleviated in strains containing a reduced amount of Hsf1 but aggravated in hsp70 deletion strains. In conclusion, we propose that the fission yeast UBL/BAG proteins release Hsf1 from Hsp70, leading to constitutive Hsf1 activation and growth defects.







Similar content being viewed by others
References
Alberti S, Demand J, Esser C, Emmerich N, Schild H, Hohfeld J (2002) Ubiquitylation of BAG-1 suggests a novel regulatory mechanism during the sorting of chaperone substrates to the proteasome. J Biol Chem 277:45920–45927
Anckar J, Sistonen L (2011) Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem 80:1089–1115
Anders S, Pyl PT, Huber W (2015) HTSeq—a python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169
Andersen KM, Jensen C, Kriegenburg F, Lauridsen AM, Gordon C, Hartmann-Petersen R (2011) Txl1 and Txc1 are co-factors of the 26S proteasome in fission yeast. Antioxid Redox Signal 14:1601–1608
Arndt V, Rogon C, Hohfeld J (2007) To be, or not to be—molecular chaperones in protein degradation. Cell Mol Life Sci 64:2525–2541
Behl C (2016) Breaking BAG: the co-chaperone BAG3 in health and disease. Trends Pharmacol Sci 37:672–688
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120
Bracher A, Verghese J (2015) The nucleotide exchange factors of Hsp70 molecular chaperones. Front Mol Biosci 2:10
Brehmer D, Rudiger S, Gassler CS, Klostermeier D, Packschies L, Reinstein J, Mayer MP, Bukau B (2001) Tuning of chaperone activity of Hsp70 proteins by modulation of nucleotide exchange. Nat Struct Biol 8:427–432
Cabrera R, Sha Z, Vadakkan TJ, Otero J, Kriegenburg F, Hartmann-Petersen R, Dickinson ME, Chang EC (2010) Proteasome nuclear import mediated by Arc3 can influence efficient DNA damage repair and mitosis in Schizosaccharomyces pombe. Mol Biol Cell 21:3125–3136
Chen D, Toone WM, Mata J, Lyne R, Burns G, Kivinen K, Brazma A, Jones N, Bahler J (2003) Global transcriptional responses of fission yeast to environmental stress. Mol Biol Cell 14:214–229
Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, Patterson C (2001) The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol 3:93–96
Cyr DM, Hohfeld J, Patterson C (2002) Protein quality control: U-box-containing E3 ubiquitin ligases join the fold. Trends Biochem Sci 27:368–375
Demand J, Alberti S, Patterson C, Hohfeld J (2001) Cooperation of a ubiquitin domain protein and an E3 ubiquitin ligase during chaperone/proteasome coupling. Curr Biol 11:1569–1577
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21
Dragovic Z, Broadley SA, Shomura Y, Bracher A, Hartl FU (2006) Molecular chaperones of the Hsp110 family act as nucleotide exchange factors of Hsp70s. EMBO J 25:2519–2528
Espinet C, de la Torre MA, Aldea M, Herrero E (1995) An efficient method to isolate yeast genes causing overexpression-mediated growth arrest. Yeast 11:25–32
Esser C, Alberti S, Hohfeld J (2004) Cooperation of molecular chaperones with the ubiquitin/proteasome system. Biochim Biophys Acta 1695:171–188
Funakoshi M, Sasaki T, Nishimoto T, Kobayashi H (2002) Budding yeast Dsk2p is a polyubiquitin-binding protein that can interact with the proteasome. Proc Natl Acad Sci U S A 99:745–750
Gotz R, Kramer BW, Camarero G, Rapp UR (2004) BAG-1 haplo-insufficiency impairs lung tumorigenesis. BMC Cancer 4:85
Hartl FU, Hayer-Hartl M (2009) Converging concepts of protein folding in vitro and in vivo. Nat Struct Mol Biol 16:574–581
Huang P, Gautschi M, Walter W, Rospert S, Craig EA (2005) The Hsp70 Ssz1 modulates the function of the ribosome-associated J-protein Zuo1. Nat Struct Mol Biol 12:497–504
Kabbage M, Dickman MB (2008) The BAG proteins: a ubiquitous family of chaperone regulators. Cell Mol Life Sci 65:1390–1402
Kettern N, Dreiseidler M, Tawo R, Hohfeld J (2010) Chaperone-assisted degradation: multiple paths to destruction. Biol Chem 391:481–489
Kriegenburg F, Jakopec V, Poulsen EG, Nielsen SV, Roguev A, Krogan N, Gordon C, Fleig U, Hartmann-Petersen R (2014) A chaperone-assisted degradation pathway targets kinetochore proteins to ensure genome stability. PLoS Genet 10:e1004140
Le Goff X, Chesnel F, Delalande O, Couturier A, Dreano S, Le GC, Vigneau C, Arlot-Bonnemains Y (2016) Aggregation dynamics and identification of aggregation-prone mutants of the von Hippel-Lindau tumor suppressor protein. J Cell Sci 129:2638–2650
Luders J, Demand J, Hohfeld J (2000) The ubiquitin-related BAG-1 provides a link between the molecular chaperones Hsc70/Hsp70 and the proteasome. J Biol Chem 275:4613–4617
Lyne R, Burns G, Mata J, Penkett CJ, Rustici G, Chen D, Langford C, Vetrie D, Bahler J (2003) Whole-genome microarrays of fission yeast: characteristics, accuracy, reproducibility, and processing of array data. BMC Genomics 4:27
Madsen L, Schulze A, Seeger M, Hartmann-Petersen R (2007) Ubiquitin domain proteins in disease. BMC Biochem 8(Suppl 1):S1
Mathiassen SG, Larsen IB, Poulsen EG, Madsen CT, Papaleo E, Lindorff-Larsen K, Kragelund BB, Nielsen ML, Kriegenburg F, Hartmann-Petersen R (2015) A two-step protein quality control pathway for a misfolded DJ-1 variant in fission yeast. J Biol Chem 290:21141–21153
Matsuyama A, Shirai A, Yashiroda Y, Kamata A, Horinouchi S, Yoshida M (2004) pDUAL, a multipurpose, multicopy vector capable of chromosomal integration in fission yeast. Yeast 21:1289–1305
McClellan AJ, Tam S, Kaganovich D, Frydman J (2005) Protein quality control: chaperones culling corrupt conformations. Nat Cell Biol 7:736–741
Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194:795–823
Murata S, Minami Y, Minami M, Chiba T, Tanaka K (2001) CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep 2:1133–1138
Nielsen SV, Poulsen EG, Rebula CA, Hartmann-Petersen R (2014) Protein quality control in the nucleus. Biomolecules 4:646–661
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817–2826
Papadakis ES, Barker CR, Syed H, Reeves T, Schwaiger S, Stuppner H, Troppmair J, Blaydes JP, Cutress RI (2016) The bag-1 inhibitor, Thio-2, reverses an atypical 3D morphology driven by bag-1 L overexpression in a MCF-10A model of ductal carcinoma in situ. Oncogenesis 5:e215
Penney M, Samejima I, Wilkinson CR, McInerny CJ, Mathiassen SG, Wallace M, Toda T, Hartmann-Petersen R, Gordon C (2012) Fission yeast 26S proteasome mutants are multi-drug resistant due to stabilization of the Pap1 transcription factor. PLoS One 7:e50796
Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE (2009) Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem 78:959–991
Saito Y, Takeda J, Okada M, Kobayashi J, Kato A, Hirota K, Taoka M, Matsumoto T, Komatsu K, Isobe T (2013) The proteasome factor Bag101 binds to Rad22 and suppresses homologous recombination. Sci Rep 3:2022
Seeger M, Hartmann-Petersen R, Wilkinson CR, Wallace M, Samejima I, Taylor MS, Gordon C (2003) Interaction of the anaphase-promoting complex/cyclosome and proteasome protein complexes with multiubiquitin chain-binding proteins. J Biol Chem 278:16791–16796
Sondermann H, Scheufler C, Schneider C, Hohfeld J, Hartl FU, Moarefi I (2001) Structure of a bag/Hsc70 complex: convergent functional evolution of Hsp70 nucleotide exchange factors. Science 291:1553–1557
Stone M, Hartmann-Petersen R, Seeger M, Bech-Otschir D, Wallace M, Gordon C (2004) Uch2/Uch37 is the major deubiquitinating enzyme associated with the 26S proteasome in fission yeast. J Mol Biol 344:697–706
Takayama S, Krajewski S, Krajewska M, Kitada S, Zapata JM, Kochel K, Knee D, Scudiero D, Tudor G, Miller GJ, Miyashita T, Yamada M, Reed JC (1998) Expression and location of Hsp70/Hsc-binding anti-apoptotic protein BAG-1 and its variants in normal tissues and tumor cell lines. Cancer Res 58:3116–3131
Tang SC, Shehata N, Chernenko G, Khalifa M, Wang X (1999) Expression of BAG-1 in invasive breast carcinomas. J Clin Oncol 17:1710–1719
Vjestica A, Zhang D, Liu J, Oliferenko S (2013) Hsp70-Hsp40 chaperone complex functions in controlling polarized growth by repressing Hsf1-driven heat stress-associated transcription. PLoS Genet 9:e1003886
Wang CY, Wen WL, Nilsson D, Sunnerhagen P, Chang TH, Wang SW (2012) Analysis of stress granule assembly in Schizosaccharomyces pombe. RNA 18:694–703
Wiederkehr T, Bukau B, Buchberger A (2002) Protein turnover: a CHIP programmed for proteolysis. Curr Biol 12:R26–R28
Wilkinson CR, Seeger M, Hartmann-Petersen R, Stone M, Wallace M, Semple C, Gordon C (2001) Proteins containing the UBA domain are able to bind to multi-ubiquitin chains. Nat Cell Biol 3:939–943
Williamson DS, Borgognoni J, Clay A, Daniels Z, Dokurno P, Drysdale MJ, Foloppe N, Francis GL, Graham CJ, Howes R, Macias AT, Murray JB, Parsons R, Shaw T, Surgenor AE, Terry L, Wang Y, Wood M, Massey AJ (2009) Novel adenosine-derived inhibitors of 70 kDa heat shock protein, discovered through structure-based design. J Med Chem 52:1510–1513
Zobeck KL, Buckley MS, Zipfel WR, Lis JT (2010) Recruitment timing and dynamics of transcription factors at the Hsp70 loci in living cells. Mol Cell 40:965–975
Acknowledgements
The authors thank Mrs. Anne-Marie Bonde-Lauridsen for expert technical assistance, Dr. Colin Gordon, Dr. Shao-Win Wang, Dr. Aleksandar Vjestica, and Dr. Snezhana Oliferenko for reagents, and Dr. Klavs B. Hendil for helpful discussions and comments on the manuscript. This work has been supported financially by grants to R.H.P. from the Lundbeck Foundation, the Danish Cancer Society, the Danish Council for Independent Research (Natural Sciences), the A.P. Møller Foundation for the Advancement of Medical Science, Aase & Ejnar Danielsens Fond, and the Novo Nordisk Foundation. This article is based upon work from COST Action (PROTEOSTASIS BM1307), supported by European Cooperation in Science and Technology (COST).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Poulsen, E.G., Kampmeyer, C., Kriegenburg, F. et al. UBL/BAG-domain co-chaperones cause cellular stress upon overexpression through constitutive activation of Hsf1. Cell Stress and Chaperones 22, 143–154 (2017). https://doi.org/10.1007/s12192-016-0751-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-016-0751-z