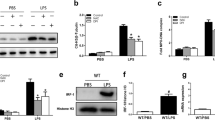
Abstract
The relationship between von Willebrand factor (VWF) and inflammation has attracted considerable attention in recent years. VWF, which is stored in the Weibel–Palade bodies (WPBs) of endothelial cells (ECs), is released from WPBs in response to inflammatory stimuli and is thought to contribute to inflammation by promoting leukocyte extravasation. In this study, lung injury model mice were produced by intratracheal injection with lipopolysaccharides. The severity of lung inflammation was evaluated in mice with different genotypes (wild-type, Vwf−/−, Adamts13−/−) and mice treated with drugs that inhibit VWF function. Lung inflammation was significantly ameliorated in Vwf−/− mice compared with wild-type mice. Furthermore, inflammation was significantly suppressed in wild-type mice treated with anti-VWF A1 antibody or recombinant human ADAMTS13 compared with the untreated control group. The underlying mechanism appears to be an increased VWF/ADAMTS13 ratio at the site of inflammation and the interaction between blood cell components, such as leukocytes and platelets, and the VWF A1 domain, which promotes leukocyte infiltration into the lung. This study suggested that ADAMTS13 protein and other VWF-targeting agents may be a novel therapeutic option for treatment of pulmonary inflammatory diseases.






Similar content being viewed by others
Data availability
The datasets generated in the current study are available from the corresponding author on reasonable request.
References
Soejima K, Mimura N, Hirashima M, Maeda H, Hamamoto T, Nakagaki T, et al. A novel human metalloprotease synthesized in the liver and secreted into the blood: possibly, the von Willebrand factor-cleaving protease? J Biochem. 2001;130:475–80.
Shida Y, Nishio K, Sugimoto M, Mizuno T, Hamada M, Kato S, et al. Functional imaging of shear-dependent activity of ADAMTS13 in regulating mural thrombus growth under whole blood flow conditions. Blood. 2008;111:1295–8.
Soejima K, Nakamura H, Hirashima M, Morikawa W, Nozaki C, Nakagaki T. Analysis on the molecular species and concentration of circulating ADAMTS13 in Blood. J Biochem. 2006;139:147–54.
Soejima K, Nakagaki T. Interplay between ADAMTS13 and von Willebrand factor in inherited and acquired thrombotic microangiopathies. Semin Hematol. 2005;42:56–62.
O’Brien M. The reciprocal relationship between inflammation and coagulation. Top Companion Anim Med. 2012;27:46–52.
Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med. 2010;38:S26-34.
Pendu R, Terraube V, Christophe OD, Gahmberg CG, de Groot PG, Lenting PJ, et al. P-selectin glycoprotein ligand 1 and beta2-integrins cooperate in the adhesion of leukocytes to von Willebrand factor. Blood. 2006;108:3746–52.
Denis CV, André P, Saffaripour S, Wagner DD. Defect in regulated secretion of P-selectin affects leukocyte recruitment in von Willebrand factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:4072–7.
Hillgruber C, Steingräber AK, Pöppelmann B, Denis CV, Ware J, Vestweber D, et al. Blocking von Willebrand factor for treatment of cutaneous inflammation. J Invest Dermatol. 2014;134:77–86.
Denis C, Methia N, Frenette PS, Rayburn H, Ullman-Culleré M, Hynes RO, et al. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc Natl Acad Sci. 1998;95:9524–9.
Banno F, Kokame K, Okuda T, Honda S, Miyata S, Kato H, et al. Complete deficiency in ADAMTS13 is prothrombotic, but it alone is not sufficient to cause thrombotic thrombocytopenic purpura. Blood. 2006;107:3161–6.
Parry GC, Erlich JH, Carmeliet P, Luther T, Mackman N. Low levels of tissue factor are compatible with development and hemostasis in mice. J Clin Invest. 1998;101:560–9.
Pedersen B, Holscher T, Sato Y, Pawlinski R, Mackman N. A balance between tissue factor and tissue factor pathway inhibitor is required for embryonic development and hemostasis in adult mice. Blood. 2005;105:2777–82.
Soejima K, Matsumoto M, Kokame K, Yagi H, Ishizashi H, Maeda H, et al. ADAMTS-13 cysteine-rich/spacer domains are functionally essential for von Willebrand factor cleavage. Blood. 2003;102:3232–7.
Shima M, Morimoto J, Imai S, Tsubura Y, Yoshioka A, Fukui H. Production and characterization of monoclonal antibodies against von Willebrand factor (vWF). Blood & Vessel. 1985;16:205–7.
Fujimura Y, Usami Y, Titani K, Niinomi K, Nishio K, Takase T, et al. Studies on anti-von Willebrand factor (vWF) monoclonal antibody NMC-4, which inhibits both ristocetin- and botrocetin-induced vWF binding to platelet glycoprotein Ib. Blood. 1991;77:113–20.
Celikel R, Varughese KI, Madhusudan, Yoshioka A, Ware J, Ruggeri ZM. Crystal structure of the von Willebrand factor A1 domain in complex with the function blocking NMC-4 Fab. Nat Struct Biol. 1998;5:189–94.
Kato S, Matsumoto M, Matsuyama T, Isonishi A, Hiura H, Fujimura Y. Novel monoclonal antibody-based enzyme immunoassay for determining plasma levels of ADAMTS13 activity. Transfusion. 2006;46:1444–52.
Chun CD, Liles WC, Frevert CW, Glenny RW, Altemeier WA. Mechanical ventilation modulates Toll-like receptor-3-induced lung inflammation via a MyD88-dependent, TLR4-independent pathway: a controlled animal study. BMC Pulm Med. 2010;10:57.
Bastarache JA, Sebag SC, Clune JK, Grove BS, Lawson WE, Janz DR, et al. Low levels of tissue factor lead to alveolar haemorrhage, potentiating murine acute lung injury and oxidative stress. Thorax. 2012;67:1032–9.
Petri B, Broermann A, Li H, Khandoga AG, Zarbock A, Krombach F, et al. von Willebrand factor promotes leukocyte extravasation. Blood. 2010;116:4712–9.
Fujioka M, Hayakawa K, Mishima K, Kunizawa A, Irie K, Higuchi S, et al. ADAMTS13 gene deletion aggravates ischemic brain damage: a possible neuroprotective role of ADAMTS13 by ameliorating postischemic hypoperfusion. Blood. 2010;115:1650–3.
Fujioka M, Nakano T, Hayakawa K, Irie K, Akitake Y, Sakamoto Y, et al. ADAMTS13 gene deletion enhances plasma high-mobility group box1 elevation and neuroinflammation in brain ischemia-reperfusion injury. Neurol Sci. 2012;33:1107–15.
De Meyer SF, Savchenko AS, Haas MS, Schatzberg D, Carroll MC, Schiviz A, et al. Protective anti-inflammatory effect of ADAMTS13 on myocardial ischemia/reperfusion injury in mice. Blood. 2012;120:5217–23.
Doi M, Matsui H, Takeda H, Saito Y, Takeda M, Matsunari Y, et al. ADAMTS13 safeguards the myocardium in a mouse model of acute myocardial infarction. Thromb Haemost. 2012;108:1236–8.
Gandhi C, Motto DG, Jensen M, Lentz SR, Chauhan AK. ADAMTS13 deficiency exacerbates VWF-dependent acute myocardial ischemia/reperfusion injury in mice. Blood. 2012;120:5224–30.
Schiviz A, Wuersch K, Piskernik C, Dietrich B, Hoellriegl W, Rottensteiner H, et al. A new mouse model mimicking thrombotic thrombocytopenic purpura: correction of symptoms by recombinant human ADAMTS13. Blood. 2012;119:6128–35.
Pillai VG, Bao J, Zander CB, McDaniel JK, Chetty PS, Seeholzer SH, et al. Human neutrophil peptides inhibit cleavage of von Willebrand factor by ADAMTS13: a potential link of inflammation to TTP. Blood. 2016;128:110–9.
Bernardo A, Ball C, Nolasco L, Moake JF, Dong J-F. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood. 2004;104:100–6.
Fu X, Chen J, Gallagher R, Zheng Y, Chung DW, López JA. Shear stress–induced unfolding of VWF accelerates oxidation of key methionine residues in the A1A2A3 region. Blood. 2011;118:5283–91.
Wang Y, Chen J, Ling M, López JA, Chung DW, Fu X. Hypochlorous acid generated by neutrophils inactivates ADAMTS13: an oxidative mechanism for regulating ADAMTS13 proteolytic activity during inflammation. J Biol Chem. 2015;290:1422–31.
Zheng L, Zhang D, Cao W, Song W-C, Zheng XL. Synergistic effects of ADAMTS13 deficiency and complement activation in pathogenesis of thrombotic microangiopathy. Blood. 2019;134:1095–105.
Turner NA, Moake J. Assembly and activation of alternative complement components on endothelial cell-anchored ultra-large von Willebrand factor links complement and hemostasis-thrombosis. PLoS ONE. 2013;8: e59372.
Acknowledgements
We thank Drs. Shogo Kasuda (Department of Legal Medicine, Nara Medical University, Japan) and Koichi Kokame (National Cerebral and Cardiovascular Center, Japan) for their technical advice.
Author information
Authors and Affiliations
Contributions
Conceptualization: YO and KT; investigation: YO, YT, RK, RM, CO, and KT; formal analysis: YO and KT; data curation: YO and KT; writing—original draft: YO; writing—review and editing: YO, SM, CH. YT, AS, RK, RM, CO, KN, MS, KS, NM, MS, KT.
Corresponding author
Ethics declarations
Conflict of interest
AS, RK, KT and MS: members of Medicinal Biology of Thrombosis and Hemostasis established by Nara Medical University and Chugai Pharmaceutical Co., Ltd. RK: an employee of Chugai Pharmaceutical Co., Ltd. a stock owner of the company. KS: an employee of KM Biologics Co., Ltd. MS: patents for inventions relating to products of Chugai Pharmaceutical Co., Ltd.; representative of Medicinal Biology of Thrombosis and Hemostasis collaborative research laboratory; research support from Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd. and CSL Behring.; honoraria or consultation fees from Chugai Pharmaceutical Co., Ltd.; speaker’s bureau from Chugai Pharmaceutical Co., Ltd., CSL Behring, Sanofi, Bayer, Novo Nordisk Pharma, Takeda Pharmaceutical Co., Ltd., Pfizer and Fujimoto Seiyaku Corp. YO, SM, CH, YT, RM, CO, KN, MS, and NM: no conflict of financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Onodera, Y., Mitani, S., Hosoda, C. et al. Regulation of von Willebrand factor by ADAMTS13 ameliorates lipopolysaccharide-induced lung injury in mice. Int J Hematol 118, 699–710 (2023). https://doi.org/10.1007/s12185-023-03668-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-023-03668-x