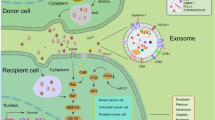
Abstract
Hematopoiesis is maintained and regulated by a bone marrow-specific microenvironment called a niche. In hematological malignancies, tumor cells induce niche remodeling, and the reconstructed niche is closely linked to disease pathogenesis. Recent studies have suggested that extracellular vesicles (EVs) secreted from tumor cells play a principal role in niche remodeling in hematological malignancies. Although EVs are emerging as potential therapeutic targets, the underlying mechanism of action remains unclear, and selective inhibition remains a challenge. This review summarizes remodeling of the bone marrow microenvironment in hematological malignancies and its contribution to pathogenesis, as well as roles of tumor-derived EVs, and provides a perspective on future research in this field.



Similar content being viewed by others
Data availability
No new data were created in this article.
References
Mikkola HKA, Orkin SH. The journey of developing hematopoietic stem cells. Development (Camb, Engl). 2006;133(19):3733–44. https://doi.org/10.1242/dev.02568.
Méndez-Ferrer S, Bonnet D, Steensma DP, Hasserjian RP, Ghobrial IM, Gribben JG, et al. Bone marrow niches in haematological malignancies. Nat Rev Cancer. 2020;20(5):285–98. https://doi.org/10.1038/s41568-020-0245-2.
Karantanou C, Minciacchi VR, Karantanos T. Extracellular vesicles in myeloid neoplasms. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms23158827.
Hayashi Y, Kawabata KC, Tanaka Y, Uehara Y, Mabuchi Y, Murakami K, et al. MDS cells impair osteolineage differentiation of MSCs via extracellular vesicles to suppress normal hematopoiesis. Cell Rep. 2022. https://doi.org/10.1016/j.celrep.2022.110805.
Yoshioka Y, Katsuda T, Ochiya T. Extracellular vesicles and encapusulated miRNAs as emerging cancer biomarkers for novel liquid biopsy. Jpn J Clin Oncol. 2018;48(10):869–76. https://doi.org/10.1093/jjco/hyy120.
Tkach M, Thery C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–32. https://doi.org/10.1016/j.cell.2016.01.043.
Ohyashiki JH, Umezu T, Ohyashiki K. Extracellular vesicle-mediated cell-cell communication in haematological neoplasms. Philos Trans R Soc Lond B Biol Sci. 2018;373(1737):20160484. https://doi.org/10.1098/rstb.2016.0484.
Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. 2019;49(3):347–60. https://doi.org/10.1016/j.devcel.2019.04.011.
Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20(8):833–46. https://doi.org/10.1038/nm.3647.
Lymperi S, Ferraro F, Scadden DT. The HSC niche concept has turned 31. Ann N Y Acad Sci. 2010;1192(1):12–8. https://doi.org/10.1111/j.1749-6632.2009.05223.x.
Wang LD, Wagers AJ. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat Rev Mol Cell Biol. 2011;12(10):643–55. https://doi.org/10.1038/nrm3184.
Itkin T, Gur-Cohen S, Spencer JA, Schajnovitz A, Ramasamy SK, Kusumbe AP et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. 2016;advance online publication. https://doi.org/10.1038/nature17624. http://www.nature.com/nature/journal/vaop/ncurrent/abs/nature17624.html#supplementary-information.
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–6. https://doi.org/10.1038/nature02040.
Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103(9):3258–64. https://doi.org/10.1182/blood-2003-11-4011.
Bowers M, Zhang B, Ho Y, Agarwal P, Chen CC, Bhatia R. Osteoblast ablation reduces normal long-term hematopoietic stem cell self-renewal but accelerates leukemia development. Blood. 2015;125(17):2678–88. https://doi.org/10.1182/blood-2014-06-582924.
Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–62. https://doi.org/10.1038/nature10783.
Greenbaum A, Hsu Y-MS, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227. https://doi.org/10.1038/nature11926. https://www.nature.com/articles/nature11926#supplementary-information.
Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33(3):387–99. https://doi.org/10.1016/j.immuni.2010.08.017.
Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–36. https://doi.org/10.1016/j.cell.2007.08.025.
Nakamura Y, Arai F, Iwasaki H, Hosokawa K, Kobayashi I, Gomei Y, et al. Isolation and characterization of endosteal niche cell populations that regulate hematopoietic stem cells. Blood. 2010;116(9):1422–32. https://doi.org/10.1182/blood-2009-08-239194.
Joseph C, Quach Julie M, Walkley Carl R, Lane Steven W, Lo Celso C, Purton LE. Deciphering hematopoietic stem cells in their niches: a critical appraisal of genetic models, lineage tracing, and imaging strategies. Cell Stem Cell. 2013;13(5):520–33. https://doi.org/10.1016/j.stem.2013.10.010.
Hanoun M, Zhang D, Mizoguchi T, Pinho S, Pierce H, Kunisaki Y, et al. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell. 2014;15(3):365–75. https://doi.org/10.1016/j.stem.2014.06.020.
Forte D, García-Fernández M, Sánchez-Aguilera A, Stavropoulou V, Fielding C, Martín-Pérez D, et al. Bone marrow mesenchymal stem cells support acute myeloid leukemia bioenergetics and enhance antioxidant defense and escape from chemotherapy. Cell Metab. 2020. https://doi.org/10.1016/j.cmet.2020.09.001.
Arranz L, Sanchez-Aguilera A, Martin-Perez D, Isern J, Langa X, Tzankov A, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512(7512):78–81. https://doi.org/10.1038/nature13383.
Sawada K, Sato N, Notoya A, Tarumi T, Hirayama S, Takano H, et al. Proliferation and differentiation of myelodysplastic CD34+ cells: phenotypic subpopulations of marrow CD34+ cells. Blood. 1995;85(1):194–202.
Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464(7290):852–7. https://doi.org/10.1038/nature08851.
Zambetti NA, Ping Z, Chen S, Kenswil KJG, Mylona MA, Sanders MA, et al. Mesenchymal inflammation drives genotoxic stress in hematopoietic stem cells and predicts disease evolution in human pre-leukemia. Cell Stem Cell. 2016;19(5):613–27. https://doi.org/10.1016/j.stem.2016.08.021.
Boocock GR, Morrison JA, Popovic M, Richards N, Ellis L, Durie PR, et al. Mutations in SBDS are associated with Shwachman–Diamond syndrome. Nat Genet. 2003;33(1):97–101. https://doi.org/10.1038/ng1062.
Kode A, Manavalan JS, Mosialou I, Bhagat G, Rathinam CV, Luo N, et al. Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature. 2014;506(7487):240–4. https://doi.org/10.1038/nature12883.
Dong L, Yu WM, Zheng H, Loh ML, Bunting ST, Pauly M, et al. Leukaemogenic effects of Ptpn11 activating mutations in the stem cell microenvironment. Nature. 2016;539(7628):304–8. https://doi.org/10.1038/nature20131.
Shiozawa Y, Havens AM, Pienta KJ, Taichman RS. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia. 2008;22(5):941–50. https://doi.org/10.1038/leu.2008.48.
Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–34. https://doi.org/10.1038/nature12984.
Bianco P. Bone and the hematopoietic niche: a tale of two stem cells. Blood. 2011;117(20):5281–8. https://doi.org/10.1182/blood-2011-01-315069.
Jacome-Galarza CE, Percin GI, Muller JT, Mass E, Lazarov T, Eitler J, et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature. 2019;568(7753):541–5. https://doi.org/10.1038/s41586-019-1105-7.
Krevvata M, Silva BC, Manavalan JS, Galan-Diez M, Kode A, Matthews BG, et al. Inhibition of leukemia cell engraftment and disease progression in mice by osteoblasts. Blood. 2014;124(18):2834–46. https://doi.org/10.1182/blood-2013-07-517219.
Frisch BJ, Ashton JM, Xing L, Becker MW, Jordan CT, Calvi LM. Functional inhibition of osteoblastic cells in an in vivo mouse model of myeloid leukemia. Blood. 2012;119(2):540–50. https://doi.org/10.1182/blood-2011-04-348151.
Kumar B, Garcia M, Weng L, Jung X, Murakami JL, Hu X, et al. Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia. 2017. https://doi.org/10.1038/leu.2017.259.
Weidner H, Rauner M, Trautmann F, Schmitt J, Balaian E, Mies A, et al. Myelodysplastic syndromes and bone loss in mice and men. Leukemia. 2017;31(4):1003–7. https://doi.org/10.1038/leu.2017.7.
Datzmann T, Trautmann F, Tesch F, Mies A, Hofbauer LC, Platzbecker U, et al. Associations of myeloid hematological diseases of the elderly with osteoporosis: a longitudinal analysis of routine health care data. Leuk Res. 2018;69:81–6. https://doi.org/10.1016/j.leukres.2018.04.010.
Krause DS, Fulzele K, Catic A, Sun CC, Dombkowski D, Hurley MP et al. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat Med. 2013;19(11):1513–7. https://doi.org/10.1038/nm.3364. http://www.nature.com/nm/journal/v19/n11/abs/nm.3364.html#supplementary-information.
Schepers K, Pietras EM, Reynaud D, Flach J, Binnewies M, Garg T, et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell. 2013;13(3):285–99. https://doi.org/10.1016/j.stem.2013.06.009.
Li AJ, Calvi LM. The microenvironment in myelodysplastic syndromes: Niche-mediated disease initiation and progression. Exp Hematol. 2017;55:3–18. https://doi.org/10.1016/j.exphem.2017.08.003.
Kim PG, Niroula A, Shkolnik V, McConkey M, Lin AE, Słabicki M, et al. Dnmt3a-mutated clonal hematopoiesis promotes osteoporosis. J Exp Med. 2021. https://doi.org/10.1084/jem.20211872.
Rohatgi N, Zou W, Collins PL, Brestoff JR, Chen TH, Abu-Amer Y, et al. ASXL1 impairs osteoclast formation by epigenetic regulation of NFATc1. Blood Adv. 2018;2(19):2467–77. https://doi.org/10.1182/bloodadvances.2018018309.
Kuek V, Hughes AM, Kotecha RS, Cheung LC. Therapeutic targeting of the leukaemia microenvironment. Int J Mol Sci. 2021;22(13):6888.
Goloviznina NA, Verghese SC, Yoon YM, Taratula O, Marks DL, Kurre P. Mesenchymal stromal cell-derived extracellular vesicles promote myeloid-biased multipotent hematopoietic progenitor expansion via toll-like receptor engagement. J Biol Chem. 2016;291(47):24607–17. https://doi.org/10.1074/jbc.M116.745653.
Morhayim J, van de Peppel J, Braakman E, Rombouts EWJC, ter Borg MND, Dudakovic A, et al. Osteoblasts secrete miRNA-containing extracellular vesicles that enhance expansion of human umbilical cord blood cells. Sci Rep. 2016;6(1):32034. https://doi.org/10.1038/srep32034.
Wen S, Dooner M, Cheng Y, Papa E, Del Tatto M, Pereira M, et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia. 2016;30(11):2221–31. https://doi.org/10.1038/leu.2016.107.
Morhayim J, Ghebes CA, Erkeland SJ, ter Borg MND, Hoogenboezem RM, Bindels EMJ, et al. Identification of osteolineage cell-derived extracellular vesicle cargo implicated in hematopoietic support. FASEB J. 2020;34(4):5435–52. https://doi.org/10.1096/fj.201902610R.
Kfoury YS, Ji F, Mazzola M, Sykes DB, Scherer AK, Anselmo A, et al. tiRNA signaling via stress-regulated vesicle transfer in the hematopoietic niche. Cell Stem Cell. 2021;28(12):2090-103.e9. https://doi.org/10.1016/j.stem.2021.08.014.
Gu H, Chen C, Hao X, Wang C, Zhang X, Li Z, et al. Sorting protein VPS33B regulates exosomal autocrine signaling to mediate hematopoiesis and leukemogenesis. J Clin Investig. 2016;126(12):4537–53. https://doi.org/10.1172/JCI87105.
Jiang J, Woulfe DS, Papoutsakis ET. Shear enhances thrombopoiesis and formation of microparticles that induce megakaryocytic differentiation of stem cells. Blood. 2014;124(13):2094–103. https://doi.org/10.1182/blood-2014-01-547927.
Shi X-F, Wang H, Kong F-X, Xu Q-Q, Xiao F-J, Yang Y-F, et al. Exosomal miR-486 regulates hypoxia-induced erythroid differentiation of erythroleukemia cells through targeting Sirt1. Exp Cell Res. 2017;351(1):74–81. https://doi.org/10.1016/j.yexcr.2016.12.023.
Jiang J, Kao C-Y, Papoutsakis ET. How do megakaryocytic microparticles target and deliver cargo to alter the fate of hematopoietic stem cells? J Control Release. 2017;247:1–18. https://doi.org/10.1016/j.jconrel.2016.12.021.
Kovuru N, Raghuwanshi S, Gutti RK. Exosome mediated differentiation of megakaryocytes: a study on TLR mediated effects. J Thromb Thrombolysis. 2019;48(1):171–3. https://doi.org/10.1007/s11239-019-01862-5.
Qu M, Zou X, Fang F, Wang S, Xu L, Zeng Q, et al. Platelet-derived microparticles enhance megakaryocyte differentiation and platelet generation via miR-1915-3p. Nat Commun. 2020;11(1):4964. https://doi.org/10.1038/s41467-020-18802-0.
Horiguchi H, Kobune M, Kikuchi S, Yoshida M, Murata M, Murase K, et al. Extracellular vesicle miR-7977 is involved in hematopoietic dysfunction of mesenchymal stromal cells via poly(rC) binding protein 1 reduction in myeloid neoplasms. Haematologica. 2016. https://doi.org/10.3324/haematol.2015.134932.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28. https://doi.org/10.1038/nrm.2017.125.
Vader P, Breakefield XO, Wood MJA. Extracellular vesicles: emerging targets for cancer therapy. Trends Mol Med. 2014;20(7):385–93. https://doi.org/10.1016/j.molmed.2014.03.002.
Peng D, Wang H, Li L, Ma X, Chen Y, Zhou H, et al. miR-34c-5p promotes eradication of acute myeloid leukemia stem cells by inducing senescence through selective RAB27B targeting to inhibit exosome shedding. Leukemia. 2018;32(5):1180–8. https://doi.org/10.1038/s41375-018-0015-2.
Marleau AM, Chen C-S, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. 2012;10(1):134. https://doi.org/10.1186/1479-5876-10-134.
Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10(5):619–24. https://doi.org/10.1038/ncb1725.
Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci. 2009;106(10):3794–9. https://doi.org/10.1073/pnas.0804543106.
del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor–bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106(5):1604–11. https://doi.org/10.1182/blood-2004-03-1095.
Mandal SK, Iakhiaev A, Pendurthi UR, Rao LVM. Acute cholesterol depletion impairs functional expression of tissue factor in fibroblasts: modulation of tissue factor activity by membrane cholesterol. Blood. 2005;105(1):153–60. https://doi.org/10.1182/blood-2004-03-0990.
Thind A, Wilson C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J Extracell Vesicles. 2016;5(1):31292. https://doi.org/10.3402/jev.v5.31292.
Lai CP, Kim EY, Badr CE, Weissleder R, Mempel TR, Tannous BA, et al. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat Commun. 2015;6:7029. https://doi.org/10.1038/ncomms8029.
Koumangoye RB, Sakwe AM, Goodwin JS, Patel T, Ochieng J. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLoS ONE. 2011;6(9):e24234. https://doi.org/10.1371/journal.pone.0024234.
Zomer A, Maynard C, Verweij FJ, Kamermans A, Schafer R, Beerling E, et al. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161(5):1046–57. https://doi.org/10.1016/j.cell.2015.04.042.
Whitley JA, Kim S, Lou L, Ye C, Alsaidan OA, Sulejmani E, et al. Encapsulating Cas9 into extracellular vesicles by protein myristoylation. J Extracell Ves. 2022;11(4):e12196. https://doi.org/10.1002/jev2.12196.
Somiya M, Kuroda SI. Engineering of extracellular vesicles for small molecule-regulated cargo loading and cytoplasmic delivery of bioactive proteins. Mol Pharm. 2022;19(7):2495–505. https://doi.org/10.1021/acs.molpharmaceut.2c00192.
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Ves. 2018;7(1):1535750. https://doi.org/10.1080/20013078.2018.1535750.
Acknowledgements
This work was supported by AMED (22gm6210022h0003), JSPS KAKENHI (20H00537 (DI), 20K21622 (DI), 20J01911(YH), 21K16258(YH)), a grant from SENSHIN Medical Research Foundation (DI), TERUMO LIFE SCIENCE FOUNDATION (DI), Daiwa Securities Health Foundation (DI), The MEXT Joint Research Center program, Yokohama City University (DI), and Nippon Shinyaku (YH).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Hayashi, Y., Nishimura, K., Tanaka, A. et al. Extracellular vesicle-mediated remodeling of the bone marrow microenvironment in myeloid malignancies. Int J Hematol 117, 821–829 (2023). https://doi.org/10.1007/s12185-023-03587-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-023-03587-x