Abstract
Reactivation of Epstein–Barr virus (EBV) has been considered a very rare event among patients on immunomodulatory drugs (IMiDs) such as lenalidomide, and an association between the two has not well been recognized. We have recently experienced a rare case of multiple myeloma in which the patient had suffered EBV reactivation during long-term lenalidomide maintenance therapy. The patient subsequently developed EBV-associated lymphoproliferative disease (LPD) as well as EBV-associated hemophagocytic lymphohistiocytosis (EBV–HLH), which was fatal despite intensive treatment. Although rare, clinicians should be aware that such fatal EBV reactivation could occur as a minor yet critical complication of long-term maintenance therapy with IMiDs in multiple myeloma patients. Regular monitoring and early detection of EBV reactivation would be beneficial for these patients, so that proper diagnostic examinations can be initiated without delay.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epstein–Barr virus (EBV) is one of the most prevalent viruses among humans, and approximately 90 to 95 percent of adults are EBV antibody seropositive [1]. Like other members of the herpesvirus family, EBV has a latency phase. However, unlike other herpesvirus, the principal human host cells for EBV are limited to B lymphocytes, T lymphocytes, NK cells, epithelial cells and myocytes. Chronic infection of EBV could sometimes transform these cells and develop various epithelial cell or hematological malignancies, especially among patients with immunosuppressive conditions [2]. Studies show that initial infection followed by lytic EBV replication and latent EBV infection could both contribute to the malignant transformation of the EBV-infected cells [3]. Interestingly, immunomodulatory drugs (IMiDs) such as lenalidomide have been shown to promote reactivation of EBV lytic replication in mammalian cells in vitro [4]. Under the presence of lenalidomide, substrate selectivity of cereblon, the key molecule that mediates protein degradation via the ubiquitin–proteasome systems, is directed toward a limited number of targets including transcription factors such as Ikaros (IKZF1) and Aiolos (IKZF3) [5]. Because Ikaros has been known as an indirect repressor of genes that are required for the activation of EBV [6], degradation of such transcription factor by lenalidomide treatment could lead to the reactivation of EBV in mammalian cells. Despite these findings from basic biological experiments, the development of EBV reactivation is very rare among patients on IMiDs treatment, and thus the association of lenalidomide use and EBV reactivation has not been well-recognized in clinical settings.
We have recently experienced a rare case of multiple myeloma who had suffered EBV reactivation during the long-term maintenance therapy with lenalidomide for 4 years. The patient subsequently developed EBV-associated lymphoproliferative disease (EBV-LPD) as well as hemophagocytic lymphohistiocytosis (HLH), which was fatal despite intensive treatment. Although rare, reactivation of EBV could occur in patients with multiple myeloma under long-term IMiDs treatment. Periodical monitoring and early detection of EBV reactivation could be beneficial for such patients to initiate proper diagnostic examinations without delay.
Case description
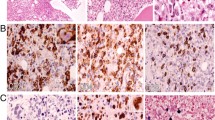
A 75-year-old Japanese male with a 4-year history of non-secretary multiple myeloma (international staging system I, international staging system-revised II), presented with malaise and periodical fever during the 45th course of the lenalidomide maintenance therapy. The patient had been in a stringent complete response after three courses of induction chemotherapy of bortezomib, lenalidomide, and dexamethasone (VRd), followed by autologous hematopoietic stem cell transplantation and lenalidomide maintenance therapy. During the initial workup, mild pancytopenia (white blood cell (WBC) count: 1500/µL, hemoglobin level: 118 g/L, platelet count: 1.3 × 105/µL) and inflammation (C-reactive protein (CRP): 1.2 × 104 µg/L) were noted. Positron emission tomography combined with computed tomography (PET-CT) showed significantly increased uptake of fluorodeoxyglucose (FDG) in the multiple lymph nodes systemically, as well as in the pituitary gland, the liver, and the spleen (Fig. 1A, B; Day 0 in Fig. 2). Extramedullary plasmacytoma or lymphoproliferative disease (LPD) was suspected, and the fine-needle aspiration biopsy (FNA/FNB) was performed. The biopsied lymph node did not contain enough B cells to examine their clonality by southern blotting or flow cytometry. After a 1-month follow-up in the outpatient department, the patient was admitted to the hospital for further investigations (1st admission in Fig. 2). Besides persistent fever above 38 ℃, the elevation of CRP to 1.3 × 105 µg/L, liver dysfunction (aspartate aminotransferase (AST) 122 IU/L, alanine aminotransferase (ALT) 103 IU/L, lactate dehydrogenase (LDH) 381 IU/L, γ-glutamyl transpeptidase (γ-GTP) 462 IU/L, alkaline phosphatase (ALP) 279 IU/L, total bilirubin 29.1 µmol/L), and progressive pancytopenia (WBC counts of 1000/µL, a hemoglobin level of 94 g/L, platelet counts of 4.7 × 104/µL) were noted. Results of physical examination and systemic imaging study with CT scan were negative for active bacterial or fungal infections. Notably, the serological antibody test for EBV resulted positive for VCA (virus capsid antigen)-IgG (320 ×) and EA (early antigen)-IgG (80 ×), while negative for EBNA (EBV nuclear antibody)-IgG. Purification of viral nucleic acids was performed using the QIA symphony DSP Virus/Pathogen Mini Kit version 1 (Qiagen). TaqMan gene expression assay of EBV–DNA was carried out using the TaqPath qPCR Master Mix, CG (Applied Biosystems). The reaction was carried out for 50 cycles of two-step PCR (denaturation at 94 ℃ for 15 s, and annealing/extension at 60 ℃ for 60 s). The fluorescence intensity was analyzed by the QuantStudio 12 K Flex Real-Time PCR System (Applied Biosystems). The whole blood EBV–DNA level was elevated to 900 copies/mL, indicating the reactivation of EBV infection. The symptoms gradually improved with supportive care and termination of the lenalidomide maintenance therapy for multiple myeloma, and the patient was discharged (Discharge in Fig. 2). Within 1 month after the discharge, the patient was readmitted to the hospital because of the aggravation of malaise and fever (2nd Admission in Fig. 2). Complete blood counts showed recurrence of prominent pancytopenia with decreased WBC counts of 700/µL (Neutrophil 500/µL), a hemoglobin level of 111 g/L, and platelet counts of 5.9 × 104/µL. Elevations of the serum CRP level to 1.1 × 105 µg/L, the serum ferritin level to 5.1 × 103 ng/mL, and soluble interleukin-2 receptor levels to 1.4 × 104 U/mL were observed. Persistent liver dysfunction (AST 115 IU/L, ALT 116 IU/L, LDH 377 IU/L, γ-GTP 913 IU/L, ALP 673 IU/L) with hyperbilirubinemia (total bilirubin 90.6 µmol/L, and direct bilirubin 57 µmol/L) was noted as well. Laboratory data at 3 months before the 1st admission, at the 1st and 2nd admission is shown in Supplemental Table 1. Imaging studies with CT scan showed hepatosplenomegaly, bilateral pleural effusion and ascites. In addition, the serum EBV-DNA level was significantly increased to 9.0 × 103 copies/mL. To examine the cell-lineage-specific infection of EBV, we sorted the hematopoietic cells into five lineages− CD19 + B cells, CD4 + T-cells, CD8 + T-cells, CD56 + NK cells and other cells–and extracted genomic DNA from each of the lineages. Quantification of the levels of EBV genomic DNA by the polymerase chain reaction (PCR) analysis showed specific amplification exclusively from the CD19 + B cells (1.1 × 103 copies/μg DNA). Together with proliferated EBV-positive B cells, multiple lymph node enlargement with active FDG incorporation by PET–CT, and increased levels of serum EBV–DNA, the present case was clinically diagnosed as EBV–LPD. Analysis of the bone marrow aspiration showed hypocellular bone marrow with a significant proliferation of phagocyting macrophages, as well as emergence of a few large lymphoid cells with basophilic cytoplasm (Fig. 1C, D). Considering the persistent pancytopenia, elevated ferritin level of 17,484 ng/mL, and hypertriglyceridemia of 3.7 mmol/L, the patient was also diagnosed as HLH based on HLH-2004 diagnostic criteria [7], in addition to EBV–LPD. With a hope to ameliorate severe pancytopenia, the patient immediately underwent HLH-2004 regimen, which includes continuous use of cyclosporine and dexamethasone with periodical use of etoposide [8]. The pancytopenia and immunosuppression, however, rapidly progressed despite intensive therapy, and the patient succumbed to death due to invasive pulmonary aspergillosis and multi-organ dysfunction on Day 25 of the HLH-2004 regimen. The autopsy has confirmed that the resolution of multiple lymph node enlargement followed by replacement with scarring fibrosis, indicating that the EBV-infected lymphocytes have been eradicated after administration of intensive HLH-2004 chemotherapy. The clinical course of the patient is summarized in Fig. 2.
Development of EBV–LPD and EBV–HLH following long-term lenalidomide treatment for multiple myeloma. A PET–CT showed increased uptake of FDG in multiple lymph nodes, pituitary gland, liver, and spleen (SUV max: 20.5). B, C Analysis of the bone marrow aspiration showed hypocellular bone marrow with significant proliferation of phagocyting macrophages indicated by arrowheads (B) and infiltration of a few large lymphoid cells with basophilic cytoplasm indicated by arrows (C)
The clinical course of the case. The patient developed EBV-associated lymphoproliferative disease (LPD) as well as EBV-associated hemophagocytic lymphohistiocytosis (EBV-HLH) after 4 years of lenalidomide maintenance therapy for multiple myeloma. Despite intensive treatment including HLH-2004 regimen, pancytopenia and immunosuppression did not improve and proved lethal. ALT aspartate alanine transferase, BMA/B bone marrow aspiration/biopsy, EBV-DNA EB virus deoxyribonucleic acid, G-CSF granulocyte colony-stimulating factor, HLH hemophagocytic lymphohistiocytosis, PET-CT positron emission tomography-computed tomography, Plt platelets, CRP C-reactive protein, WBC white blood cells
Discussion
EBV–LPD is a life-threatening complication in patients under immunosuppressive conditions. Because the immunodeficiency often disrupts the normal balance between latency infected B-cell proliferation and the EBV-specific T-cell response, the EBV-positive B cells outgrow and may develop into LPD [9]. Iatrogenic immunodeficiency is often induced by methotrexate and other well-known immunosuppressive medications in patients with autoimmune disease or post-organ transplant. Spontaneous development of EBV–LPD has repeatedly been documented in these immunocompromised patients [10]. Of note, novel agents with immunomodulatory effects have recently been shown to induce such chronic immunodeficiency and lymphoproliferative diseases [11, 12]. Lenalidomide, a derivative of thalidomide, is a potent anti-myeloma agent as well as a representative immunomodulatory drug. Although the present case might be the first and only report describing the clear association of lenalidomide use and EBV–LPD development, reactivation of EBV was seen in one out of the 30 patients with multiple myeloma under lenalidomide maintenance therapy in the HOVON76 Trial, and EBV–LPD was observed in one Japanese patient out of 96 East Asian patients after administration of a combination therapy of daratumumab, lenalidomide, and dexamethasone, which resulted in discontinuation of the therapy in the POLLUX study, both of which were regarded as an unrelated adverse event at that time [13, 14]. In clinical settings, it is difficult to tell replication of EBV, a condition that infectious virion is actively produced during lytic replication, from proliferation of EBV-infected B cells stimulated by latent EBV proteins. Based on the finding of high levels of serum VCA–IgG in the present patient, we speculate that reactivation of EBV is more likely in this case [15]. Considering the present case and the results from HOVON76 trial and POLLUX study, potential association of LPD development and IMiDs use is suspected, and its incidence might have been underestimated, which should be further investigated in future studies.
Besides developing EBV–LPD after long-term lenalidomide use, the present case progressed to HLH, which is usually a non-neoplastic, benign process with prominent but self-limited hemophagocytosis. In contrast, HLH is an often fatal, progressive condition with uncontrollable hemophagocytosis. The present case reminds hematologists in practice that reactivation of EBV could happen in patients under long-term IMiDs treatment, and periodical monitoring and early detection of EBV reactivation are critical in such patients to initiate proper management of this potentially fatal condition.
Data availability
Relevant clinical data is available upon request.
References
Okuno Y, Murata T, Sato Y, Muramatsu H, Ito Y, Watanabe T, et al. Defective Epstein-Barr virus in chronic active infection and haematological malignancy. Nat Microbiol. 2019;4(3):404–13.
Cohen JI, Kimura H, Nakamura S, Ko YH, Jaffe ES. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8–9 September 2008. Ann Oncol. 2009;20(9):1472–82.
Ma SD, Hegde S, Young KH, Sullivan R, Rajesh D, Zhou Y, et al. A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas. J Virol. 2011;85(1):165–77.
Jones RJ, Iempridee T, Wang X, Lee HC, Mertz JE, Kenney SC, et al. Lenalidomide, thalidomide, and pomalidomide reactivate the Epstein-Barr virus lytic cycle through phosphoinositide 3-kinase signaling and Ikaros expression. Clin Cancer Res. 2016;22(19):4901–12.
Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, et al. The Myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2013;343(6168):305–9.
Iempridee T, Reusch JA, Riching A, Johannsen EC, Dovat S, Kenney SC, et al. Epstein-Barr virus utilizes Ikaros in regulating its latent-lytic switch in B cells. J Virol. 2014;88(9):4811–27.
Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–31.
Bergsten E, Horne A, Aricó M, Astigarraga I, Egeler RM, Filipovich AH, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130(25):2728–38.
Münz C. Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat Rev Microbiol. 2019;17(11):691–700.
Natkunam Y, Gratzinger D, Chadburn A, Goodlad JR, Chan JKC, Said J, et al. Immunodeficiency-associated lymphoproliferative disorders: time for reappraisal? Blood. 2018;132(18):1871–8.
Pina-Oviedo S, Miranda RN, Jeffrey L, Medeiros M. Cancer therapy-associated lymphoproliferative disorders: an under-recognized type of immunodeficiency-associated lymphoproliferative disorder. Am J Surg Pathol. 2018;42(1):116–29.
Daroontum T, Kohno K, Eladl AE, Satou A, Sakakibara A, Matsukage S, et al. Comparison of Epstein-Barr virus-positive mucocutaneous ulcer associated with treated lymphoma or methotrexate in Japan. Histopathology. 2018;72(7):1115–27.
Kneppers E, van der Holt B, Kersten MJ, Zweegman S, Meijer E, Huls G, et al. Lenalidomide maintenance after nonmyeloablative allogeneic stem cell transplantation in multiple myeloma is not feasible: results of the HOVON 76 Trial. Blood. 2011;118(9):2413–9.
Suzuki K, Dimopoulos MA, Takezako N, Okamoto S, Shinagawa A, Matsumoto M, et al. Daratumumab, lenalidomide, and dexamethasone in East Asian patients with relapsed or refractory multiple myeloma: subgroup analyses of the phase 3 POLLUX study. Blood Cancer J. 2018. https://doi.org/10.1038/s41408-018-0071-x.
Jog NR, Young KA, Munroe ME, Harmon MT, Guthridge JM, Kelly JA, et al. Association of Epstein-Barr virus serological reactivation with transitioning to systemic lupus erythematosus in at-risk individuals. Ann Rheum Dis. 2019;78(9):1235–41.
Author information
Authors and Affiliations
Contributions
MY collected data and wrote the manuscript. KM supervised research and wrote the manuscript, which was reviewed and edited by the other authors. MJ and MN analyzed the bone marrow aspiration samples. YK, AI, and TU collected the histopathological data. KI performed cell lineage-specific amplification and quantification of EBV. YY, HF, AH, and HM participated in the discussion and gave critical comments on research direction. MK supervised research and gave final approval for submission.
Corresponding author
Ethics declarations
Conflict of interest
M.K. received research funding from Pfizer, Otsuka Pharmaceutical, Chugai Pharmaceutical, Astellas, Kyowa Kirin, Takeda Pharmaceutical, Teijin, Eisai, Sumitomo Dainippon Pharma, Nippon Shinyaku, AbbVie, Daiichi Sankyo and Ono Pharmaceutical; advisory fees from Kyowa Kirin, Celgene, Chugai Pharmaceutical and MSD; and lecture fees from MSD, Astellas, Otsuka Pharmaceutical, Ono Pharmaceutical, Celgene, Daiichi Sankyo, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Chugai Pharmaceutical, Janssen Pharmaceutical, Kyowa Kirin, AbbVie, Pfizer, AstraZeneca, Bristol-Myers Squibb, Amgen, Sanwa Kagaku, Sanofi, SymBio Pharmaceutical and Nippon Shinyaku. M.J. reports lecture fees from Bristol-Myers Squibb K.K.; and lecture fees from Novartis Pharma K.K. None of these are related to the current study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Yoshida, M., Morita, K., Fukushima, H. et al. Development of Epstein–Barr virus-associated lymphoproliferative disorder and hemophagocytic lymphohistiocytosis during long-term lenalidomide maintenance therapy in multiple myeloma. Int J Hematol 117, 769–773 (2023). https://doi.org/10.1007/s12185-022-03499-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-022-03499-2