Abstract
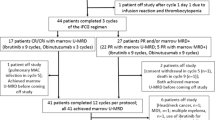
Fludarabine-cyclophosphamide-rituximab (FCR) has been the gold standard front-line treatment for fit CLL patients until novel agent’s introduction. Decision between either time-limited FCR or “endless” Bruton’s tyrosine kinase inhibitor (BTKi) therapy may be difficult in fit IGHV-mutated-non-TP53 cases. We describe the outcomes after front-line FCR in 110 CLL patients from 5 centres in Catalonia, Spain, over a period of more than 10 years. ORR was 96.3% and CR 74.5%. Median second-treatment free survival (TFS1) was 6.2 years and median OS was 10.8 years. 50 (45.5%) patients required a subsequent therapy. Median third-treatment free survival was better for BTKi than for chemotherapy ± antiCD20 strategies (not reached vs 3.1 years, p = 0.003). Only 50 (45.5%) patients completed 6 cycles of FCR, and the main reason for discontinuation was cytopenia 29 (26.4%). 15 (13.6%) patients developed a second cancer, and 5 (4.5%) patients experienced a Richter's transformation (RT). At the end of follow-up, 50 (45.5%) patients remained in CR. Response rates, TFS1, OS, RT, and second cancers did not differ between patients treated with 6 vs 4 cycles of FCR. In conclusion, front-line FCR treatment leads to very long CR in almost half of patients, and BTKi yields excellent outcomes in relapsed patients.




Similar content being viewed by others
References
Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, International Group of Investigators, German Chronic Lymphocytic Leukaemia Study Group, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–74.
Eichhorst B, Robak T, Montserrat E, Ghia P, Niemann CU, Kater AP, SMO Guidelines Committee, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol. 2021;32(1):23–33.
Thompson PA, Tam CS, O’Brien SM, Wierda WG, Stingo F, Plunkett W, et al. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood. 2016;127(3):303–9.
Fischer K, Bahlo J, Fink AM, Goede V, Herling CD, Cramer P, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208–15.
Keating MJ, O’Brien S, Albitar M, Lerner S, Plunkett W, Giles F, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–88.
Benjamini O, Jain P, Trinh L, Qiao W, Strom SS, Lerner S, et al. Second cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: distribution and clinical outcomes. Leuk Lymphoma. 2015;56(6):1643–50.
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, RESONATE-2 Investigators, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–37.
Burger JA, Barr PM, Robak T, Owen C, Ghia P, Tedeschi A, et al. Long-term efficacy and safety of first line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787–98.
Shanafelt TD, Wang XV, Hanson CA, Paietta EM, O’Brien S, Barrientos JC, et al. Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: updated results of the E1912 Trial. Blood. 2022;140:112–20. https://doi.org/10.1182/blood.2021014960.
Fischer K, Al-Sawaf O, Bahlo J, Fink AM, Tandon M, Dixon M, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225–36.
Al-Sawaf O, Zhang C, Tandon M, Sinha A, Fink AM, Robrecht S, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21(9):1188–200.
Herishanu Y, Goldschmidt N, Bairey O, Ruchlemer R, Fineman R, Rahimi-Levene N, et al. Israeli CLL Study Group. Efficacy and safety of front-line therapy with fludarabine-cyclophosphamide-rituximab regimen for chronic lymphocytic leukemia outside clinical trials: the Israeli CLL Study Group experience. Haematologica. 2015;100(5):662–9.
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. IwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–60.
Cramer P, Fink AM, Busch R, Eichhorst B, Wendtner CM, Pflug N, et al. Second-line therapies of patients initially treated with fludarabine and cyclophosphamide or fludarabine, cyclophosphamide and rituximab for chronic lymphocytic leukemia within the CLL8 protocol of the German CLL Study Group. Leuk Lymphoma. 2013;54(8):1821–2.
Tam CS, O’Brien S, Plunkett W, Wierda W, Ferrajoli A, Wang X, et al. Long-term results of first salvage treatment in CLL patients treated initially with FCR (fludarabine, cyclophosphamide, rituximab). Blood. 2014;124(20):3059–64.
Munir T, Brown JR, O’Brien S, Barrientos JC, Barr PM, Reddy NM, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353–63.
Al-Sawaf O, Zhang C, Lu T, Liao MZ, Panchal A, Robrecht S, et al. Minimal residual disease dynamics after venetoclax-obinutuzumab treatment: extended off-treatment follow-up from the randomized CLL14 Study. J Clin Oncol. 2021;39(36):4049–60.
Stilgenbauer S, Schnaiter A, Paschka P, Zenz T, Rossi M, Döhner K, et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: results from the CLL8 trial. Blood. 2014;123(21):3247–54.
Rossi D, Gaidano G. The clinical implications of gene mutations in chronic lymphocytic leukaemia. Br J Cancer. 2016;114(8):849–54.
Kater AP, Wu JQ, Kipps T, Eichhorst B, Hillmen P, D’Rozario J, et al. Venetoclax plus rituximab in relapsed chronic lymphocytic leukemia: 4-year results and evaluation of impact of genomic complexity and gene mutations from the MURANO phase III study. J Clin Oncol. 2020;38(34):4042–54.
Author information
Authors and Affiliations
Contributions
ACO: Honoraria for consultancy from Janssen-Cilag, Abbvie, Alexion; for speaker from Janssen-Cilag, Abbvie, AstraZeneca, Alexion, Takeda, Roche and for travel from Janssen-Cilag, Abbvie, Alexion. JMR: Honoraria for consultancy from Janssen-Cilag; for speaker from Abbvie, Janssen-Cilag, AstraZeneca, Roche, Gilead and for travel from Abbvie, Janssen-Cilag, Roche. CF: Honoraria for speaker from Abbvie, AstraZeneca, Janssen. MR-L: Honoraria for travel from Janssen-Cilag. ED-D: Honoraria for consultancy from Takeda, Roche; for speaker from Takeda and for travel from BMS-Cellgene, Roche. AS: Honoraria for consultancy from MSD, Roche, Janssen, Astra Zeneca Takeda, Pierre Fabre, GenMab, BMS Celgene, Kite, Novartis, Janssen, Sanofi and for speaker from Pierre Fabre, Takeda, MSD, BMS/Celgene, Novartis, Gilead Kite, Sanofi, GenMab. EG-B: Honoraria for consultancy from Janssen-Cilag, Abbvie, Gilead, Kiowa, EUSAPharma, Incyte, Lilly, Beigene, Novartis; for speaker from Janssen-Cilag, Abbvie, Takeda, Roche, EUSAPharma, Incyte and for travel from Janssen-Cilag, Abbvie, Roche, EUSAPharma.
Corresponding author
Ethics declarations
Competing interests
Janilson Do Nascimento, David Gallardo, Maite Encuentra, Patricia López, Josep Maria Ribera and Josep Sarrá declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Oliveira, A.C., Roncero, J.M., Ferrá, C. et al. Front-line fludarabine-cyclophosphamide-rituximab (FCR) in 110 patients with chronic lymphocytic leukaemia (CLL): real-life experience with long-term outcomes, toxicities and responses to second-line therapies. Int J Hematol 117, 388–397 (2023). https://doi.org/10.1007/s12185-022-03488-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-022-03488-5