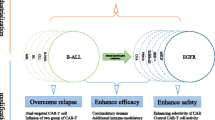
Abstract
Chimeric antigen receptor-T (CAR-T) cell therapy has shown significant therapeutic efficacy in the treatment of hematological B-cell malignancies. However, the efficacy of CAR-T cell therapy against solid tumors is limited due to the heterogeneity of tumor antigens and the immunosuppressive tumor microenvironment. Therefore, there is strong demand for novel technologies to improve the efficacy of CAR-T cell therapy. In addition, as CAR-T cells often cause severe side effects, systems to control the activity of CAR-T cells so as to avoid or lessen the occurrence and intensity of these side effects are needed. Here, we describe recently emerging approaches to enhance and/or regulate CAR-T cell functions. These approaches have led to the development of CAR-T therapies with improved efficacy and safety, which are expected to be clinically applied to a variety of cancer types in combination with other therapies, such as immune checkpoint inhibitors, chemotherapy, molecular targeted drugs, and radiation therapy.



Similar content being viewed by others
References
Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor modified T cells in chronic lymphoid leukemia. NEngl J Med. 2011;365:725–33. https://doi.org/10.1056/NEJMoa1103849.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48. https://doi.org/10.1056/NEJMoa1709866.
Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–95.
Grant SJ, Rosko AE, Giri S, et al. Clinical presentation, risk factors, and outcomes of immune effector cell-associated neurotoxicity syndrome following chimeric antigen receptor T cell therapy: a systematic review. Transplant Cell Ther. 2022;28(6):294–302.
Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–51.
Labanieh L, Majzner RG, Mackall CL. Programming CAR-T cells to kill cancer. Nat Biomed Eng. 2018;2:377–91.
Batra SA, Rathi P, Guo L, Bulsara S, et al. Glypican-3-specific CAR T cells co-expressing IL15 and IL21 have superior expansion and antitumor activity against hepatocellular carcinoma. Cancer Immunol Res. 2020;8(3):309–20.
Hu B, Ren J, June CH, et al. Augmentation of antitumor immunity by human and mouse CAR T cells secreting IL-18. Cell Rep. 2017;20:3025–33.
Pegram HJ, Lee JC, Brentjens RJ, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119:4133–41.
Liu Y, Di S, Jiang H, et al. Armored inducible expression of IL-12 enhances antitumor activity of glypican-3-targeted chimeric antigen receptor-engineered T cells in hepatocellular carcinoma. J Immunol. 2019;203:198–207.
Tamada K, Geng D, Sakoda Y, Bansal N, Srivastava R, Li Z, Davila E. Redirecting gene-modified T cells toward various cancer types using tagged antibodies. Clin Cancer Res. 2012;18(23):6436–45.
Urbanska K, Lanitis E, Kelderman S, et al. A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res. 2012;72:1844–52.
Cho JH, Collins JJ, Wong WW. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell. 2018;173(6):1426–38.
Rodgers DT, Kim CH, Young TS, et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc Natl Acad Sci USA. 2016;113(4):E459–68.
John LB, Devaud C, Duong CPM, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19:5636–46.
Choi BD, Carter BS, Maus MV, et al. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J Immunother Cancer. 2019;7:304.
Suarez ER, Chang DK, Marasco WA, et al. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget. 2016;7:34341–55.
Rafiq S, Miele MM, Li Z, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol. 2018;36:847–56.
Nakajima M, Nagano H, Tamada K, et al. Improved survival of chimeric antigen receptor-engineered T (CAR-T) and tumor-specific T cells caused by anti-programmed cell death protein 1 single-chain variable fragment-producing CAR T cells. Cancer Sci. 2019;110:3079–88.
Narayan V, Fraietta JA, Haas NB, et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nature Med. 2022;28:724–34.
Adachi K, Sakoda Y, Tamada K, et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol. 2018;36:346–51.
Di Stasi A, Tey SK, Grilley B, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–83.
Tiberghien P, Ferrand C, Deconinck E, et al. Administration of herpes simplex-thymidine kinase-expressing donor T cells with a T cell-depleted allogeneic marrow graft. Blood. 2001;97:63–72.
Griffioen M, van Egmond EHM, Kester MGD, Willemze R, Falkenburg JHF, Heemskerk MHM. Retroviral transfer of human CD20 as a suicide gene for adoptive T-cell therapy. Haematologica. 2009;94:1316–20.
Wang X, Chang W-C, Ostberg JR, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118:1255–63.
Paszkiewicz PJ, Fräßle SP, Drexler I, et al. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J Clin Invest. 2016;126:4262–72.
Sakemura R, Terakura S, Miyao K, et al. A Tet-On inducible system for controlling CD19-chimeric antigen receptor expression upon drug administration. Cancer Immunol Res. 2016;4:658–68.
Zimmermann K, Abken H, Schambach A, et al. Design and characterization of an “all-in-one” lentiviral vector system combining constitutive anti-G(D2) CAR expression and inducible cytokines. Cancers. 2020;12:375.
Roybal KT, Park JS, Lim WA. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell. 2016;164:770–9.
Wilkie S, van Schalkwyk MC, Maher J, et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clin Immunol. 2012;32:1059–70.
Fedorov VD, Themeli M, Sadelain M, et al. PD-1–and CTLA-4–based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5:215ra172.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KT and YS is shareholder at Noile-Immune Biotech Inc., and receive remuneration from Noile-Immune Biotech Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ohta, K., Sakoda, Y. & Tamada, K. Novel technologies for improving the safety and efficacy of CAR-T cell therapy. Int J Hematol 117, 647–651 (2023). https://doi.org/10.1007/s12185-022-03478-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-022-03478-7