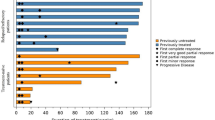
Abstract
In this study, the efficacy and safety of filgrastim biosimilar (F-BS) were retrospectively compared to those of filgrastim original in the treatment of malignant lymphoma with CHASE (± R) or DeVIC(± R) in 78 patients. The median number of filgrastim doses was 11 in the F-BS group and 8 in the filgrastim group after CHASE (± R) (p = 0.8), and 10 in the F-BS group and 10 in the filgrastim group after DeVIC (± R) (p = 0.45). The median days until neutrophil recovery to ≥ 1000/μL was 10 days with F-BS versus 10 days with filgrastim after CHASE ± R (p = 0.59), and 9 days with F-BS versus 10 days with filgrastim after DeVIC ± R (p = 0.828). Febrile neutropenia (FN) was observed in 5 patients (41.7%) in the F-BS group and 9 (52.9%) in the filgrastim group after CHASE ± R therapy (p = 0.616), and in 11 patients (36.7%) in the F-BS group and 9 (47.4%) in the filgrastim group after DeVIC ± R (p = 0.462). The present results suggest that the efficacy and safety of F-BS are comparable to those of filgrastim original, with no significant differences in clinical factors. Use of F-BS also reduced medical costs per course of CHASE ± R therapy by 170.22 US dollars.

Similar content being viewed by others
References
Sagara Y, Sato K, Fukuma E, Higaki K, Mizutani M, Osaki A, et al. The efficacy and safety of FSK0808, filgrastim biosimilar: a multicenter, non-randomized study in Japanese patients with breast cancer. Jpn J Clin Oncol. 2013;43:865–73.
Kiyama S, Kimura Y, Sakoda K, Nakamura M, Hamasaki S, Miura H, et al. Investigation of the effectiveness of an original filgrastim drug and a biosimilar. J Jpn Soc Hosp Pharm. 2015;51:867–70.
Egi M, Ogura H, Yatabe T, Atagi K, Inoue S, Iba T, et al. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic shock 2020 (J-SSCG2020). J Jpn Soc Intensive Care Med. 2021; 28.
Becker PS, Griffiths EA, Alwan LM, Bachiashvili K, Brown A, Cool R, et al. NCCN guidelines insights: hematopoietic growth factors, version 1.2020. J Natl Compr Canc Netw. 2020;18:12–22.
Kubo K, Miyazaki Y, Murayama T, Shimazaki R, Usui N, Urabe A, et al. A randomized, double-blind trial of pegfilgrastim versus filgrastim for the management of neutropenia during CHASE(R) chemotherapy for malignant lymphoma. Br J Haematol. 2016;174:563–70.
Oki Y, Ogura M, Kato H, Kikuchi A, Taji H, Kagami Y, et al. Phase II study of a salvage regimen using cyclophosphamide, high-dose cytarabine, dexamethasone, etoposide, and rituximab in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. Cancer Sci. 2008;99:179–84.
Ohtsubo T, Nakagawa Y, Fujita M, Ishida Y, Sawada S, Tomogane K, et al. Comparison between filgrastim biosimilar “Mochida” and filgrastim in malignant lymphoma: a prospective, randomized crossover comparative study. Jpn J Pharm Health Care Sci. 2015;41:793–8.
https://www.mhlw.go.jp/english/. Accessed 16 Aug 2022.
Acknowledgements
The authors would like to thank all of the physicians, nurses, pharmacists, and support personnel for their care of patients in this study.
Author information
Authors and Affiliations
Contributions
RS and HY analyzed the extracted data, contributed to writing the paper, and were involved in the treatment; ST and DI reviewed the study design and contributed to writing the paper; IN, YK, HF, GY, NU, and MH were involved in the treatment and contributed to writing the paper.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no conflict of financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Shimano, R., Yamamoto, H., Nasu, I. et al. Comparison between filgrastim biosimilar and filgrastim original for the management of neutropenia after salvage chemotherapy for malignant lymphoma. Int J Hematol 116, 856–862 (2022). https://doi.org/10.1007/s12185-022-03438-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-022-03438-1