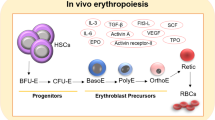
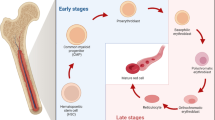
Abstract
Enucleation is a crucial event during the erythropoiesis, implicating drastic morphologic and transcriptomic/proteomic changes. While many genes deletion lead to failed or impaired enucleation have been identified, directly triggering the erythroid maturation, particularly enucleation, is still challenging. Inducing enucleation at the desired timing is necessary to develop efficient methods to generate mature, fully functional red blood cells in vitro for future transfusion therapies. However, there are considerable differences between primary erythroid cells and cultured cell sources, particularly pluripotent stem cell-derived erythroid cells and immortalized erythroid cell lines. For instance, the difference in the proliferative status between those cell types could be a critical factor, as cell cycle exit is closely connected to the terminal maturation of primary. In this review, we will discuss previous findings on the enucleation machinery and current challengings to trigger the enucleation of infinite erythroid cell sources.



Similar content being viewed by others
References
Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14: e1002533.
Schechter AN. Hemoglobin research and the origins of molecular medicine. Blood. 2008;2008(112):3927–38.
Migliaccio AR, Masselli E, Varricchio L, Whitsett C. Ex-vivo expansion of red blood cells: how real for transfusion in humans? Blood Rev. 2012;26:81–95.
D’Alessandro A, Dzieciatkowska M, Nemkov T, Hansen KC. Red blood cell proteomics update: is there more to discover? Blood Transfus. 2017;15:182–7.
Huisjes R, Bogdanova A, van Solinge WW, Schiffelers RM, Kaestner L, van Wijk R. Squeezing for life—properties of red blood cell deformability. Front Physiol. 2018;9:656.
Sankaran VG, Ludwig LS, Sicinska E, Xu J, Bauer DE, Eng JC, et al. Cyclin D3 coordinates the cell cycle during differentiation to regulate erythrocyte size and number. Genes Dev. 2012;26(18):2075–87.
Simpson CF, Kling JM. The mechanism of denucleation in circulating erythroblasts. J Cell Biol. 1967;35(1):237–45.
Awai M, Okada S, Takebayashi J, Kubo T, Inoue M, Seno S. Studies on the mechanism of denucleation of the erythroblast. Acta Haematol. 1968;39(4):193–202.
Yoshida H, Kawane K, Koike M, Mori Y, Uchiyama Y, Nagata S. Phosphatidylserine-dependent engulfment by macrophages of nuclei from erythroid precursor cells. Nature. 2005;437:754–8.
Zermati Y, Garrido C, Amsellem S, Fishelson S, Bouscary D, Valensi F, et al. Caspase activation is required for terminal erythroid differentiation. J Exp Med. 2001;193:247–54.
Boehm D, Mazurier C, Giarratana MC, Darghouth D, Faussat AM, Harmand L, et al. Caspase-3 is involved in the signalling in erythroid differentiation by targeting late progenitors. PLoS One. 2013;8: e62303.
Carlile GW, Smith DH, Wiedmann M. Caspase-3 has a nonapoptotic function in erythroid maturation. Blood. 2004;103:4310–6.
Zhao B, Mei Y, Schipma MJ, Roth EW, Bleher R, Rappoport JZ, et al. Nuclear condensation during mouse erythropoiesis requires caspase-3-mediated nuclear opening. Dev Cell. 2016;36:498–510.
Zhen R, Moo C, Zhao Z, Chen M, Feng H, Zheng X, et al. Wdr26 regulates nuclear condensation in developing erythroblasts. Blood. 2020;135:208–19.
Hattangadi SM, Martinez-Morilla S, Patterson HC, Shi J, Burke K, Avila-Figueroa A, et al. Histones to the cytosol: exportin 7 is essential for normal terminal erythroid nuclear maturation. Blood. 2014;124:1931–40.
Figueroa AA, Fasano JD, Martinez-Morilla S, Venkatesan S, Kupfer G, Hattangadi SM. miR-181a regulates erythroid enucleation via the regulation of Xpo7 expression. Haematologica. 2018;103:e341–4.
Popova EY, Krauss SW, Short SA, Lee G, Villalobos J, Etzell J, et al. Chromatin condensation in terminally differentiating mouse erythroblasts does not involve special architectural proteins but depends on histone deacetylation. Chromosome Res. 2009;17:47–64.
Ji P, Yeh V, Ramirez T, Murata-Hori M, Lodish HF. Histone deacetylase 2 is required for chromatin condensation and subsequent enucleation of cultured mouse fetal erythroblasts. Haematologica. 2010;95(12):2013–21.
Wang Y, Li W, Schulz VP, Zhao H, Qu X, Qi Q, et al. Impairment of human terminal erythroid differentiation by histone deacetylase 5 deficiency. Blood. 2021;138(17):1615–27.
Yang XJ, Seto E. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene. 2007;26:5310–8.
Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov. 2014;13:673–91.
Ling T, Crispino JD. GATA1 mutations in red cell disorders. IUBMB Life. 2020;72:106–18.
Gnanapragasam MN, Bieker JJ. Orchestration of late events in erythropoiesis by KLF1/EKLF. Curr Opin Hematol. 2017;24:183–90.
Nuez B, Michalovich D, Bygrave A, Ploemacher R, Grosveld F. Defective haematopoiesis in fetal liver resulting from inactivation of the EKLF gene. Nature. 1995;375:316–8.
Gnanapragasam MN, McGrath KE, Catherman S, Xue L, Palis J, Bieker JJ. EKLF/KLF1-regulated cell cycle exit is essential for erythroblast enucleation. Blood. 2016;128:1631–41.
Yang CT, Ma R, Axton RA, Jackson M, Taylor AH, Fidanza A, et al. Activation of KLF1 enhances the differentiation and maturation of red blood cells from human pluripotent stem cells. Stem Cells. 2017;35:886–97.
Ribeil JA, Zermati Y, Vandekerckhove J, Cathelin S, Kersual J, Dussiot M, et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 2007;445:102–5.
Tyrkalska SD, Pérez-Oliva AB, Rodríguez-Ruiz L, Martínez-Morcillo FJ, Alcaraz-Pérez F, Martínez-Navarro FJ, et al. Inflammasome regulates hematopoiesis through cleavage of the master erythroid transcription factor GATA1. Immunity. 2019;51:50–63.
Ma WB, Wang XH, Li CY, Tian HH, Zhang J, Bi JJ, et al. GPS2 promotes erythroid differentiation by control of the stability of EKLF protein. Blood. 2020;135:2302–15.
Skutelsky E, Danon D. Comparative study of nuclear expulsion from the late erythroblast and cytokinesis. Exp Cell Res. 1970;60:427–36.
Guertin DA, Trautmann S, McCollum D. Cytokinesis in eukaryotes. Microbiol Mol Biol Rev. 2002;66:155–78.
Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87.
Wang J, Ramirez T, Ji P, Jayapal SR, Lodish HF, Murata-Hori M. Mammalian erythroblast enucleation requires PI3K-dependent cell polarization. J Cell Sci. 2012;125:340–9.
Rayment I. Kinesin and myosin: molecular motors with similar engines. Structure. 1996;4:501–4.
Kobayashi I, Ubukawa K, Sugawara K, Asanuma K, Guo YM, Yamashita J, et al. Erythroblast enucleation is a dynein-dependent process. Exp Hematol. 2016;44:247–56.
Thom CS, Traxler EA, Khandros E, Nickas JM, Zhou OY, Lazarus JE, et al. Trim58 degrades Dynein and regulates terminal erythropoiesis. Dev Cell. 2014;30:688–700.
Koury ST, Koury MJ, Bondurant MC. Cytoskeletal distribution and function during the maturation and enucleation of mammalian erythroblasts. J Cell Biol. 1989;109:3005–13.
Ubukawa K, Guo YM, Takahashi M, Hirokawa M, Michishita Y, Nara M, et al. Enucleation of human erythroblasts involves non-muscle myosin IIB. Blood. 2012;119:1036–44.
Wickrema A, Koury ST, Dai CH, Krantz SB. Changes in cytoskeletal proteins and their mRNAs during maturation of human erythroid progenitor cells. J Cell Physiol. 1994;160:417–26.
Ji P, Jayapal SR, Lodish HF. Enucleation of cultured mouse fetal erythroblasts requires Rac GTPases and mDia2. Nat Cell Biol. 2008;10:314–21.
Ubukawa K, Goto T, Asanuma K, Sasaki Y, Guo YM, Kobayashi I, et al. Cdc42 regulates cell polarization and contractile actomyosin rings during terminal differentiation of human erythroblasts. Sci Rep. 2020;10:11806.
Nowak RB, Papoin J, Gokhin DS, Casu C, Rivella S, Lipton JM, et al. Tropomodulin 1 controls erythroblast enucleation via regulation of F-actin in the enucleosome. Blood. 2017;130:1144–55.
Li X, Mei Y, Yan B, Vitriol E, Huang S, Ji P, et al. Histone deacetylase 6 regulates cytokinesis and erythrocyte enucleation through deacetylation of formin protein mDia2. Haematologica. 2017;102:984–94.
Sadoul K, Wang J, Diagouraga B, Vitte AL, Buchou T, Rossini T, et al. HDAC6 controls the kinetics of platelet activation. Blood. 2012;120:4215–8.
Keerthivasan G, Small S, Liu H, Wickrema A, Crispino JD. Vesicle trafficking plays a novel role in erythroblast enucleation. Blood. 2010;116:3331–40.
Keerthivasan G, Liu H, Gump JM, Dowdy SF, Wickrema A, Crispino JD. A novel role for survivin in erythroblast enucleation. Haematologica. 2012;97:1471–9.
An C, Huang Y, Li M, Xue F, Nie D, Zhao H, et al. Vesicular formation regulated by ERK/MAPK pathway mediates human erythroblast enucleation. Blood Adv. 2021;5:4648–61.
Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112:470–8.
Soni S, Bala S, Gwynn B, Sahr KE, Peters LL, Hanspal M. Absence of erythroblast macrophage protein (Emp) leads to failure of erythroblast nuclear extrusion. J Biol Chem. 2006;281:20181–9.
Chow A, Huggins M, Ahmed J, Hashimoto D, Lucas D, Kunisaki Y, et al. CD169+ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med. 2013;19:429–36.
Wei Q, Boulais PE, Zhang D, Pinho S, Tanaka M, Frenette PS. Maea expressed by macrophages, but not erythroblasts, maintains postnatal murine bone marrow erythroblastic islands. Blood. 2019;133:1222–32.
Zhao B, Mei Y, Yang J, Ji P. Erythropoietin-regulated oxidative stress negatively affects enucleation during terminal erythropoiesis. Exp Hematol. 2016;44:975–81.
Bell AJ, Satchwell TJ, Heesom KJ, Hawley BR, Kupzig S, Hazell M, et al. Protein distribution during human erythroblast enucleation in vitro. PLoS One. 2013;8: e60300.
Gautier EF, Ducamp S, Leduc M, Salnot V, Guillonneau F, Dussiot M, et al. Comprehensive proteomic analysis of human erythropoiesis. Cell Rep. 2016;16:1470–84.
Alvarez-Dominguez JR, Zhang X, Hu W. Widespread and dynamic translational control of red blood cell development. Blood. 2017;129:619–29.
Neildez-Nguyen TM, Wajcman H, Marden MC, Bensidhoum M, Moncollin V, Giarratana MC, et al. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol. 2002;20:467–72.
Giarratana MC, Kobari L, Lapillonne H, Chalmers D, Kiger L, Cynober T, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23:69–74.
Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat Biotechnol. 2006;24(10):1255–6.
Zhang Y, Wang C, Wang L, Shen B, Guan X, Tian J, et al. Large-scale ex vivo generation of human red blood cells from cord blood CD34(+) cells. Stem Cells Transl Med. 2017;6:1698–709.
Olivier EN, Qiu C, Velho M, Hirsch RE, Bouhassira EE. Large-scale production of embryonic red blood cells from human embryonic stem cells. Exp Hematol. 2006;34:1635–42.
Dias J, Gumenyuk M, Kang H, Vodyanik M, Yu J, Thomson JA, et al. Generation of red blood cells from human induced pluripotent stem cells. Stem Cells Dev. 2011;20:1639–47.
Olivier EN, Marenah L, McCahill A, Condie A, Cowan S, Mountford JC. High-efficiency serum-free feeder-free erythroid differentiation of human pluripotent stem cells using small molecules. Stem Cells Transl Med. 2016;5:1394–405.
Lapillonne H, Kobari L, Mazurier C, Tropel P, Giarratana MC, Zanella-Cleon I, et al. Red blood cell generation from human induced pluripotent stem cells: perspectives for transfusion medicine. Haematologica. 2010;95:1651–9.
Bernecker C, Ackermann M, Lachmann N, Rohrhofer L, Zaehres H, Araúzo-Bravo MJ, et al. Enhanced ex vivo generation of erythroid cells from human induced pluripotent stem cells in a simplified cell culture system with low cytokine support. Stem Cells Dev. 2019;28:1540–51.
Kurita R, Suda N, Sudo K, Miharada K, Hiroyama T, Miyoshi H, et al. Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells. PLoS One. 2013;8: e59890.
Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–36.
Trakarnsanga K, Griffiths RE, Wilson MC, Blair A, Satchwell TJ, Meinders M. An immortalized adult human erythroid line facilitates sustainable and scalable generation of functional red cells. Nat Commun. 2017;8:14750.
Kurita R, Funato K, Abe T, Watanabe Y, Shiba M, Tadokoro K, et al. Establishment and characterization of immortalized erythroid progenitor cell lines derived from a common cell source. Exp Hematol. 2019;69:11–6.
Scully EJ, Shabani E, Rangel GW, Grüring C, Kanjee U, Clark MA, et al. Generation of an immortalized erythroid progenitor cell line from peripheral blood: a model system for the functional analysis of Plasmodium spp. invasion. Am J Hematol. 2019;94:963–74.
Daniels DE, Ferguson DCJ, Griffiths RE, Trakarnsanga K, Cogan N, MacInnes KA, et al. Reproducible immortalization of erythroblasts from multiple stem cell sources provides approach for sustainable RBC therapeutics. Mol Ther Methods Clin Dev. 2021;22:26–39.
Bagchi A, Nath A, Thamodaran V, Ijee S, Palani D, Rajendiran V, et al. Direct generation of immortalized erythroid progenitor cell lines from peripheral blood mononuclear cells. Cells. 2021;10:523.
Soboleva S, Kurita R, Kajitani N, Åkerstrand H, Miharada K. Establishment of an immortalized human erythroid cell line sustaining differentiation potential without inducible gene expression system. Hum Cell. 2022;35:408–17.
Huang X, Shah S, Wang J, Ye Z, Dowey SN, Tsang KM. Extensive ex vivo expansion of functional human erythroid precursors established from umbilical cord blood cells by defined factors. Mol Ther. 2014;22:451–63.
Hirose SI, Takayama N, Nakamura S, Nagasawa K, Ochi K, Hirata S, et al. Immortalization of erythroblasts by c-MYC and BCL-XL enables large-scale erythrocyte production from human pluripotent stem cells. Stem Cell Rep. 2013;1:499–508.
Soboleva S, Kurita R, Ek F, Åkerstrand H, Silvério-Alves R, Olsson R, et al. Identification of potential chemical compounds enhancing generation of enucleated cells from immortalized human erythroid cell lines. Commun Biol. 2021;4:677.
Acknowledgements
We thank Ryo Kurita, Martin L Olsson and Johan Flygare for discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Soboleva, S., Miharada, K. Induction of enucleation in primary and immortalized erythroid cells. Int J Hematol 116, 192–198 (2022). https://doi.org/10.1007/s12185-022-03386-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-022-03386-w