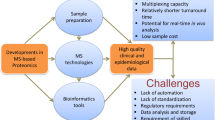
Abstract
Serum protein electrophoresis methods are widely employed to detect, quantify and isotype M-proteins for multiple myeloma patients. Increasing clinical demands to detect residual disease and interferences from new therapeutic monoclonal antibody treatments have stretched electrophoretic methods to their analytical limits. Newer techniques to detect M-proteins using mass spectrometry (MS) are emerging with improved clinical and analytical performance. These techniques are beginning to gain traction within the routine clinical lab testing. This review describes these MS methods with attention to the current and future roles such testing could play in the care of multiple myeloma patients.






Similar content being viewed by others
Abbreviations
- MM:
-
Multiple myeloma
- Ig:
-
Immunoglobulin
- SPEP:
-
Serum protein electrophoresis
- IFE:
-
Immunofixation electrophoresis
- sFLC:
-
Serum free light chain analysis
- NGF:
-
Next-generation flow cytometry
- NGS:
-
Next-generation sequencing
- t-mAbs:
-
Therapeutic monoclonal antibodies
- MS:
-
Mass spectrometry
- MRD:
-
Minimal residual disease
- miRAMM:
-
Monoclonal immunoglobulin rapid accurate mass measurements
- MALDI-TOF:
-
Matrix-assisted laser desorption ionization time of flight
References
Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564–9.
Willrich MAV, Murray DL, Kyle RA. Laboratory testing for monoclonal gammopathies: focus on monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. Clin Biochem. 2018;51:38–47.
Katzmann JA, Kyle RA, Benson J, Larson DR, Snyder MR, Lust JA, et al. Screening panels for detection of monoclonal gammopathies. Clin Chem. 2009;55(8):1517–22.
Dimopoulos M, Kyle R, Fermand JP, Rajkumar SV, San Miguel J, Chanan-Khan A, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701–5.
Flores-Montero J, Sanoja-Flores L, Paiva B, Puig N, Garcia-Sanchez O, Bottcher S, et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia. 2017;31(10):2094–103.
Martinez-Lopez J, Lahuerta JJ, Pepin F, Gonzalez M, Barrio S, Ayala R, et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123(20):3073–9.
Mills JR, Murray DL. Identification of friend or foe: the laboratory challenge of differentiating M-proteins from monoclonal antibody therapies. J Appl Lab Med: AACC Publ. 2017;1(4):421–31.
Murray D, Barnidge D. Characterization of immunoglobulin by mass spectrometry with applications for the clinical laboratory. Crit Rev Clin Lab Sci. 2013;50(4–5):91–102.
Zajec M, Langerhorst P, VanDuijn MM, Gloerich J, Russcher H, van Gool AJ, et al. Mass spectrometry for identification, monitoring, and minimal residual disease detection of M-proteins. Clin Chem. 2020;66(3):421–33.
Barnidge DR, Tschumper RC, Theis JD, Snyder MR, Jelinek DF, Katzmann JA, et al. Monitoring M-Proteins in patients with multiple myeloma using heavy-chain variable region clonotypic peptides and LC-MS/MS. J Proteom Res. 2014;13:1905.
Langerhorst P, Noori S, Zajec M, De Rijke YB, Gloerich J, van Gool AJ, et al. Multiple myeloma minimal residual disease detection: targeted mass spectrometry in blood vs next-generation sequencing in bone marrow. Clin Chem. 2021;67(12):1689–98.
Bergen HR 3rd, Dasari S, Dispenzieri A, Mills JR, Ramirez-Alvarado M, Tschumper RC, et al. Clonotypic light chain peptides identified for monitoring minimal residual disease in multiple myeloma without bone marrow aspiration. Clin Chem. 2016;62(1):243–51.
Noori S, Verkleij CPM, Zajec M, Langerhorst P, Bosman PWC, de Rijke YB, et al. Monitoring the M-protein of multiple myeloma patients treated with a combination of monoclonal antibodies: the laboratory solution to eliminate interference. Clin Chem Lab Med. 2021;59:1963.
Barnidge DR, Dasari S, Botz CM, Murray DH, Snyder MR, Katzmann JA, et al. Using mass spectrometry to monitor monoclonal immunoglobulins in patients with a monoclonal gammopathy. J Proteome Res. 2014;13:1419.
Barnidge DRKTP, Griffin TJ, Murray DL. Using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry to detect monoclonal immunoglobuli light chains in serum and urine. Rapid Commun Mass Spectrom. 2015;29:1–4.
Mills JR, Kohlhagen MC, Dasari S, Vanderboom PM, Kyle RA, Katzmann JA, et al. Comprehensive assessment of M-proteins using nanobody enrichment coupled to MALDI-TOF mass spectrometry. Clin Chem. 2016;62(10):1334–44.
Barnidge DR, Dasari S, Ramirez-Alvarado M, Fontan A, Willrich MA, Tschumper RC, et al. Phenotyping polyclonal kappa and lambda light chain molecular mass distributions in patient serum using mass spectrometry. J Proteome Res. 2014;13(11):5198–205.
Barnidge DR, Lundstrom SL, Zhang B, Dasari S, Murray DL, Zubarev RA. Subset of kappa and lambda germline sequences result in light chains with a higher molecular mass phenotype. J Proteome Res. 2015;14(12):5283–90.
Murray DL, Puig N, Kristinsson S, Usmani SZ, Dispenzieri A, Bianchi G, et al. Mass spectrometry for the evaluation of monoclonal proteins in multiple myeloma and related disorders: an international myeloma working group mass spectrometry committee report. Blood Cancer J. 2021;11(2):24.
Genzen JR, Murray DL, Abel G, Meng QH, Baltaro RJ, Rhoads DD, et al. Screening and diagnosis of monoclonal gammopathies: an international survey of laboratory practice. Arch Pathol Lab Med. 2017;142:507.
Kohlhagen M, Dasari S, Willrich M, Hetrick M, Netzel B, Dispenzieri A, et al. Automation and validation of a MALDI-TOF MS (Mass-Fix) replacement of immunofixation electrophoresis in the clinical lab. Clin Chem Lab Med. 2020;59:155.
Dasari S, Kohlhagen MC, Dispenzieri A, Willrich MAV, Snyder MR, Kourelis TV, et al. Detection of plasma cell disorders by mass spectrometry: a comprehensive review of 19,523 cases. Mayo Clin Proc. 2022;97(2):294–307.
Jimenez-Zepeda VH, Reece DE, Trudel S, Franke N, Winter A, Chen C, et al. Oligoclonal and monoclonal bands after single autologous stem cell transplant in patients with multiple myeloma: impact on overall survival and progression-free survival. Leuk Lymphoma. 2014;55(10):2284–9.
Willrich MA, Ladwig PM, Andreguetto BD, Barnidge DR, Murray DL, Katzmann JA, et al. Monoclonal antibody therapeutics as potential interferences on protein electrophoresis and immunofixation. Clin Chem Lab Med. 2016;54(6):1085–93.
Moore LM, Cho S, Thoren KL. MALDI-TOF mass spectrometry distinguishes daratumumab from M-proteins. Clin Chim Acta; Int J Clin Chem. 2019;492:91–4.
Kohlhagen MC, Mills JR, Willrich MAV, Dasari S, Dispenzieri A, Murray DL. Clearing drug interferences in myeloma treatment using mass spectrometry. Clin Biochem. 2021;92:61.
Mills JR, Kohlhagen MC, Willrich MAV, Kourelis T, Dispenzieri A, Murray DL. A universal solution for eliminating false positives in myeloma due to therapeutic monoclonal antibody interference. Blood. 2018;132(6):670–2.
Santockyte R, Puig O, Zheng N, Ouyang Z, Titsch C, Zhang YJ, et al. High-throughput therapeutic antibody interference-free high-resolution mass spectrometry assay for monitoring M-proteins in multiple myeloma. Anal Chem. 2021;93(2):834–42.
Abdallah N, Murray D, Dispenzieri A, Kapoor P, Gertz MA, Lacy MQ, et al. Tracking daratumumab clearance using mass spectrometry: implications on M protein monitoring and reusing daratumumab. Leukemia. 2022. https://doi.org/10.1038/s41375-021-01501-0.
Kirchhoff DC, Murata K, Thoren KL. Use of a daratumumab-specific immunofixation assay to assess possible immunotherapy interference at a major cancer center: our experience and recommendations. J Appl Lab Med. 2021;6:1476.
Mellors PW, Kohlhagen MC, Dasari S, Willrich MAV, Gertz MA, Kumar SK, et al. Belantamab mafodotin detection by MASS-FIX and immunofixation. Clin Chem Lab Med. 2021;59(11):e430–3.
Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354(13):1362–9.
Murray D, Kumar SK, Kyle RA, Dispenzieri A, Dasari S, Larson DR, et al. Detection and prevalence of monoclonal gammopathy of undetermined significance: a study utilizing mass spectrometry-based monoclonal immunoglobulin rapid accurate mass measurement. Blood Cancer J. 2019;9(12):102.
Milani P, Murray DL, Barnidge DR, Kohlhagen MC, Mills JR, Merlini G, et al. The utility of MASS-FIX to detect and monitor monoclonal proteins in the clinic. Am J Hematol. 2017;92(8):772–9.
Puig N, Sanfeliciano TC, Agullo Roca C, Martinez-Lopez J, Oriol A, Blanchard MJ, et al. Mass spectrometry vs immunofixation for treatment monitoring in multiple myeloma. Blood Adv. 2022. https://doi.org/10.1182/bloodadvances.2021006762.
Dispenzieri A, Krishnan A, Arendt B, Blackwell B, Wallace PK, Dasari S, et al. Mass-Fix better predicts for PFS and OS than standard methods among multiple myeloma patients participating on the STAMINA trial (BMT CTN 0702 /07LT). Blood Cancer J. 2022;12(2):27.
Giles HV, Cook MA, Drayson MT, Cook G, Wright NJ, North SJ, et al. Redefining nonmeasurable multiple myeloma using mass spectrometry. Blood. 2022;139(6):946–50.
Kohlhagen MC, Barnidge DR, Mills JR, Stoner J, Gurtner KM, Liptac AM, et al. Screening method for M-proteins in serum using nanobody enrichment coupled to MALDI-TOF mass spectrometry. Clin Chem. 2016;62(10):1345–52.
Eveillard M, Korde N, Ciardiello A, Diamond B, Lesokhin A, Mailankody S, et al. Using MALDI-TOF mass spectrometry in peripheral blood for the follow up of newly diagnosed multiple myeloma patients treated with daratumumab-based combination therapy. Clin Chim Acta; Int J Clin Chem. 2021;516:136–41.
Sepiashvili L, Kohlhagen MC, Snyder MR, Willrich MAV, Mills JR, Dispenzieri A, et al. Direct detection of monoclonal free light chains in serum by use of immunoenrichment-coupled MALDI-TOF mass spectrometry. Clin Chem. 2019;65(8):1015–22.
Abeykoon JP, Murray DL, Murray I, Jevremovic D, Otteson GE, Dispenzieri A, et al. Implications of detecting serum monoclonal protein by MASS-fix following stem cell transplantation in multiple myeloma. Br J Haematol. 2021;193(2):380–5.
Derman BA, Stefka AT, Jiang K, McIver A, Kubicki T, Jasielec JK, et al. Measurable residual disease assessed by mass spectrometry in peripheral blood in multiple myeloma in a phase II trial of carfilzomib, lenalidomide, dexamethasone and autologous stem cell transplantation. Blood Cancer J. 2021;11(2):19.
Kumar S, Murray D, Dasari S, Milani P, Barnidge D, Madden B, et al. Assay to rapidly screen for immunoglobulin light chain glycosylation: a potential path to earlier AL diagnosis for a subset of patients. Leukemia. 2019;33(1):254–7.
Dispenzieri A, Larson DR, Rajkumar SV, Kyle RA, Kumar SK, Kourelis T, et al. N-glycosylation of monoclonal light chains on routine MASS-FIX testing is a risk factor for MGUS progression. Leukemia. 2020;34(10):2749–53.
Sidana S, Murray DL, Dasari S, Go RS, Muchtar E, Willrich MA, et al. Glycosylation of immunoglobulin light chains is highly prevalent in cold agglutinin disease. Am J Hematol. 2020. https://doi.org/10.1002/ajh.25843.
Juskewitch JE, Murray JD, Norgan AP, Moldenhauer SK, Tauscher CD, Jacob EK, et al. In from the cold: M-protein light chain glycosylation is positively associated with cold agglutinin titer levels. Transfusion. 2021;61(4):1302–11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
I have potential royalties for patents licensed to the Binding Site on the use of Mass Spectrometry to detect M-proteins.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Murray, D.L. Bringing mass spectrometry into the care of patients with multiple myeloma. Int J Hematol 115, 790–798 (2022). https://doi.org/10.1007/s12185-022-03364-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-022-03364-2