Abstract
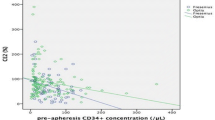
Granulocyte transfusion (GTX) is a therapeutic option for patients with prolonged neutropenia suffering from severe infections. Efficient granulocyte collection by apheresis from donors requires clear separation of granulocytes from red blood cells (RBCs), and infusion of high-molecular-weight (MW) hydroxyethyl starch (HES) facilitates RBC sedimentation. Recent research has shown that apheresis with medium-MW HES may prevent adverse effects of high-MW HES on donors, but the rationale for collection with medium-MW HES has yet to be evaluated. To validate the use of medium-MW HES, we first performed experiments with whole blood samples to determine how efficiently high-, medium- and low-MW HES separated granulocytes from RBCs, and found that medium-MW HES was just as efficient as high-MW HES. We also reviewed clinical data of granulocyte apheresis at our institution to evaluate granulocyte yields. Retrospective analysis of granulocyte collection revealed that apheresis with medium-MW HES yielded sufficient granulocytes for GTX and that donor anemia reduced collection efficiency. These results collectively may help us to establish a safer method for apheresis targeting polymorphonuclear granulocytes as an alternative to high-MW HES.


Similar content being viewed by others
References
Pizzo PA. Management of fever in patients with cancer and treatment-induced neutropenia. N Engl J Med. 1993;328(18):1323–32. https://doi.org/10.1056/NEJM199305063281808.
Robenshtok E, Gafter-Gvili A, Goldberg E, Weinberger M, Yeshurun M, Leibovici L, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25(34):5471–89. https://doi.org/10.1200/JCO.2007.12.3851.
Miles-Jay A, Butler-Wu S, Rowhani-Rahbar A, Pergam SA. Incidence rate of fluoroquinolone-resistant gram-negative rod bacteremia among allogeneic hematopoietic cell transplantation patients during an era of levofloxacin prophylaxis. Biol Blood Marrow Transplant. 2015;21(3):539–45. https://doi.org/10.1016/j.bbmt.2014.12.006.
El-Mahallawy HA, El-Wakil M, Moneer MM, Shalaby L. Antibiotic resistance is associated with longer bacteremic episodes and worse outcome in febrile neutropenic children with cancer. Pediatr Blood Cancer. 2011;57(2):283–8. https://doi.org/10.1002/pbc.22926.
Rolston KV. The use of new and better antibiotics for bacterial infections in patients with leukemia. Clin Lymphoma Myeloma. 2009;9(Suppl 3):S357–63. https://doi.org/10.3816/CLM.2009.s.035.
Peters C. Granulocyte transfusions in neutropenic patients: beneficial effects proven? Vox Sang. 2009;96(4):275–83. https://doi.org/10.1111/j.1423-0410.2008.01159.x.
Kadri SS, Remy KE, Strich JR, Gea-Banacloche J, Leitman SF. Role of granulocyte transfusions in invasive fusariosis: systematic review and single-center experience. Transfusion. 2015;55(9):2076–85. https://doi.org/10.1111/trf.13099.
Kerr JP, Liakopolou E, Brown J, Cornish JM, Fleming D, Massey E, et al. The use of stimulated granulocyte transfusions to prevent recurrence of past severe infections after allogeneic stem cell transplantation. Br J Haematol. 2003;123(1):114–8. https://doi.org/10.1046/j.1365-2141.2003.04583.x.
Price TH, Boeckh M, Harrison RW, McCullough J, Ness PM, Strauss RG, et al. Efficacy of transfusion with granulocytes from G-CSF/dexamethasone-treated donors in neutropenic patients with infection. Blood. 2015;126(18):2153–61. https://doi.org/10.1182/blood-2015-05-645986.
Zhou B, Song T, Feng Y, Zhu Z, Chang W, Liu Y, et al. Clinical outcome of granulocyte transfusion therapy for the treatment of refractory infection in neutropenic patients with hematological diseases. Ann Hematol. 2018;97(11):2061–70. https://doi.org/10.1007/s00277-018-3432-4.
Bux J, Cassens U, Dielschneider T, Duchscherer M, Edel E, Eichler H, et al. Tolerance of granulocyte donors towards granulocyte colony-stimulating factor stimulation and of patients towards granulocyte transfusions: results of a multicentre study. Vox Sang. 2003;85(4):322–5. https://doi.org/10.1111/j.0042-9007.2003.00373.x.
Strauss RG. Therapeutic granulocyte transfusions in 1993. Blood. 1993;81(7):1675–8.
Caspar CB, Seger RA, Burger J, Gmür J. Effective stimulation of donors for granulocyte transfusions with recombinant methionyl granulocyte colony-stimulating factor. Blood. 1993;81(11):2866–71.
Bensinger WI, Price TH, Dale DC, Appelbaum FR, Clift R, Lilleby K, et al. The effects of daily recombinant human granulocyte colony-stimulating factor administration on normal granulocyte donors undergoing leukapheresis. Blood. 1993;81(7):1883–8.
Liles WC, Huang JE, Llewellyn C, SenGupta D, Price TH, Dale DC. A comparative trial of granulocyte-colony-stimulating factor and dexamethasone, separately and in combination, for the mobilization of neutrophils in the peripheral blood of normal volunteers. Transfusion. 1997;37(2):182–7. https://doi.org/10.1046/j.1537-2995.1997.37297203521.x.
Stroncek DF, Yau YY, Oblitas J, Leitman SF. Administration of G-CSF plus dexamethasone produces greater granulocyte concentrate yields while causing no more donor toxicity than G–CSF alone. Transfusion. 2001;41(8):1037–44. https://doi.org/10.1046/j.1537-2995.2001.41081037.x.
Ikemoto J, Yoshihara S, Fujioka T, Ohtsuka Y, Fujita N, Kokubunji A, et al. Impact of the mobilization regimen and the harvesting technique on the granulocyte yield in healthy donors for granulocyte transfusion therapy. Transfusion. 2012;52(12):2646–52. https://doi.org/10.1111/j.1537-2995.2012.03661.x.
Cancelas JA, Padmanabhan A, Le T, Ambruso DR, Rugg N, Worsham DN, et al. Spectra Optia granulocyte apheresis collections result in higher collection efficiency of viable, functional neutrophils in a randomized, crossover, multicenter trial. Transfusion. 2015;55(4):748–55. https://doi.org/10.1111/trf.12907.
Leitner GC, Kolovratova V, Horvath M, Worel N. Granulocyte collection using a novel apheresis system eases the procedure and provides concentrates of high quality. Transfusion. 2015;55(5):991–5. https://doi.org/10.1111/trf.12928.
Janes AW, Mishler JM, Lowes B. Serial infusion effects of hydroxyethyl starch on ESR, blood typing and crossmatching and serum amylase levels. Vox Sang. 1977;32(3):131–4. https://doi.org/10.1111/j.1423-0410.1977.tb00617.x.
Lee JH, Leitman SF, Klein HG. A controlled comparison of the efficacy of hetastarch and pentastarch in granulocyte collections by centrifugal leukapheresis. Blood. 1995;86(12):4662–6.
Strauss RG, Klein HG, Leitman SF, Price TH, Lichtiger B, Martinez F, et al. Preparation of granulocyte concentrates by apheresis: collection modalities in the USA. Vox Sang. 2011;100(4):426–33. https://doi.org/10.1111/j.1423-0410.2010.01417.x.
Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–39. https://doi.org/10.1056/NEJMoa070716.
Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Åneman A, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367(2):124–34. https://doi.org/10.1056/NEJMoa1204242.
Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367(20):1901–11. https://doi.org/10.1056/NEJMoa1209759.
Auwerda JJ, Wilson JH, Sonneveld P. Foamy macrophage syndrome due to hydroxyethyl starch replacement: a severe side effect in plasmapheresis. Ann Intern Med. 2002;137(12):1013–4. https://doi.org/10.7326/0003-4819-137-12-200212170-00037.
Auwerda JJ, Leebeek FW, Wilson JH, van Diggelen OP, Lam KH, Sonneveld P. Acquired lysosomal storage caused by frequent plasmapheresis procedures with hydroxyethyl starch. Transfusion. 2006;46(10):1705–11. https://doi.org/10.1111/j.1537-2995.2006.00962.x.
Mayor S. EMA confirms that hydroxyethyl starch solutions should not be used in critically ill, sepsis, or burns patients. BMJ. 2013;347:f6197. https://doi.org/10.1136/bmj.f6197.
US Food and Drug Administration. FDA Safety Communication: Boxed Warning on increased mortality and severe renal injury, and additional warning on risk of bleeding, for use of hydroxyethyl starch solutions in some settings. 2013. http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ucm358271.htm.
European Medicines Agency. Hydroxyethyl starch solutions: CMDh introduces new measures to protect patients. 2018. https://www.ema.europa.eu/en/news/hydroxyethyl-starch-solutions-cmdh-introduces-newmeasures-protect-patients.
Gandhi SD, Weiskopf RB, Jungheinrich C, Koorn R, Miller D, Shangraw RE, et al. Volume replacement therapy during major orthopedic surgery using Voluven (hydroxyethyl starch 130/04) or hetastarch. Anesthesiology. 2007;106(6):1120–7. https://doi.org/10.1097/01.anes.0000265422.07864.37.
Nanya M, Yurugi K, Kato I, Hiramatsu H, Kawabata H, Kondo T, et al. Successful granulocyte apheresis using medium molecular weight hydroxyethyl starch. Int J Hematol. 2019;110(6):729–35. https://doi.org/10.1007/s12185-019-02755-2.
Thorausch K, Schulz M, Bialleck H, Luxembourg B, Seifried E, Bonig H. Granulocyte collections: comparison of two apheresis systems. Transfusion. 2013;53(12):3262–8. https://doi.org/10.1111/trf.12197.
Doblinger N, Bredthauer A, Mohrez M, Hähnel V, Graf B, Gruber M, et al. Impact of hydroxyethyl starch and modified fluid gelatin on granulocyte phenotype and function. Transfusion. 2019;59(6):2121–30. https://doi.org/10.1111/trf.15279.
Dullinger K, Pamler I, Brosig A, Mohrez M, Hähnel V, Offner R, et al. Granulocytapheresis with modified fluid gelatin versus high-molecular-weight hydroxyethyl starch: a matched-pair analysis. Transfusion. 2017;57(2):397–403. https://doi.org/10.1111/trf.13898.
Kamezaki K, Miyamoto T, Henzan T, Numata A, Iwasaki H, Nagafuji K, et al. Collection of mobilized peripheral blood stem cells from a donor with severe iron deficient anemia. J Clin Apher. 2007;22(5):292–4. https://doi.org/10.1002/jca.20141.
Acknowledgements
We acknowledge the technical staffs at Center for Cellular and Molecular Medicine and the clinical engineering technologists at Department of Medical Technology, Kyushu University Hospital for assistance with procedure of apheresis. We also thank the apheresis nurses at Blood Transfusion Center, Kyushu University Hospital for clinical care contributions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Henzan, T., Yamauchi, T., Yamanaka, I. et al. Granulocyte collection by polymorphonuclear cell-targeting apheresis with medium-molecular-weight hydroxyethyl starch. Int J Hematol 114, 691–700 (2021). https://doi.org/10.1007/s12185-021-03207-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03207-6