Abstract
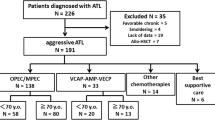
Peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS) and angioimmunoblastic T-cell lymphoma (AITL) are common subtypes of T-cell lymphoma. Although CHOP is a standard regimen for T-cell lymphoma, it has unsatisfactory outcomes. Pirarubicin is an anthracycline antibiotic with lower cardiotoxicity than doxorubicin. THP-COP (pirarubicin, cyclophosphamide, vincristine, and prednisone) is sometimes used for elderly patients with non-Hodgkin’s lymphoma in Japan. We performed a retrospective analysis using data from the population-based Osaka Cancer Registry as well as administrative data from 2010 to 2015. Of 82 enrolled patients, 51 received CHOP and 31 received THP-COP. The median age was 65 years in the CHOP group and 75 years in the THP-COP group. The probability of 3-year overall survival (OS) was 49.0% in the CHOP group and 44.9% in the THP-COP group. In the propensity score-adjusted analysis, there was no significant difference between the THP-COP and CHOP groups in the OS of the total sample [hazard ratio (HR) 0.46, 95% CI 0.14–1.55, P = 0.2]. Although our study was limited by its retrospective nature, it showed that clinical outcomes with the THP-COP regimen were comparable to those with the CHOP regimen in PTCL-NOS and AITL. Our findings should be re-assessed in larger studies in the future.


Similar content being viewed by others
References
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90.
Non-Hodgkin’s Lymphoma Classification Project. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89(11):3909–18.
Umezawa H, Takahashi Y, Kinoshita M, Naganawa H, Masuda T, Ishizuka M, et al. Tetrahydropyranyl derivatives of daunomycin and adriamycin. J Antibiot. 1979;32(10):1082–4.
Kunimoto S, Miura K, Umezawa K, Xu C-Z, Masuda T, Takeuchi T, et al. Cellular uptake and efflux and cytostatic activity of 4′-O-tetrahydropyranyladriamycin in adriamycin-sensitive and resistant tumor cell lines. J Antibiot. 1984;37(12):1697–702.
Imura H, Ito N, Shirakawa S, Sobue R, Maruyama F, Kojima H, et al. THP-COP, BHAC-VMP alternating chemotherapy in patients with non-Hodgkin’s lymphoma. Rinsho Ketsueki. 1988;29(5):688–93.
Shibata Y, Hara T, Kasahara S, Yamada T, Sawada M, Mabuchi R, et al. CHOP or THP-COP regimens in the treatment of newly diagnosed peripheral T-cell lymphoma, not otherwise specified: a comparison of doxorubicin and pirarubicin. Hematol Oncol. 2017;35(2):163–71.
Niitsu N, Umeda M. Response and adverse drug reactions to combination chemotherapy in elderly patients with aggressive non-Hodgkin’s lymphoma: comparison of CHOP, COP-BLAM, COP-BLAM III, and THP-COPBLM. Eur J Haematol. 1999;63(5):337–44.
Mori M, Kitamura K, Masuda M, Hotta T, Miyazaki T, Miura AB, et al. Long-term results of a multicenter randomized, comparative trial of modified CHOP versus THP-COP versus THP-COPE regimens in elderly patients with non-Hodgkin’s lymphoma. Int J Hematol. 2005;81(3):246–54.
Takamatsu Y, Suzumiya J, Utsunomiya A, Maeda K, Matsuoka H, Suzushima H, et al. THP-COP regimen for the treatment of peripheral T-cell lymphoma and adult T-cell leukemia/lymphoma: a multicenter phase II study. Eur J Haematol. 2010;84(5):391–7.
Tomita N, Kodama F, Tsuyama N, Sakata S, Takeuchi K, Ishibashi D, et al. Biweekly THP-COP therapy for newly diagnosed peripheral T-cell lymphoma patients. Hematol Oncol. 2015;33(1):9–14.
Fuji S, Kida S, Morishima T, Nakata K, Miyashiro I, Ishikawa J. Clinical outcomes of patients with adult T Cell leukemia-lymphoma in a nonendemic metropolitan area: a retrospective analysis of the population-based Osaka cancer registry. Biol Blood Marrow Transplant. 2020;26(8):1433–8.
Morishima T, Matsumoto Y, Koeda N, Shimada H, Maruhama T, Matsuki D, et al. Impact of comorbidities on survival in gastric, colorectal, and lung cancer patients. J Epidemiol. 2019;29(3):110–5.
Shigemi D MT, Shibata A, Tabuchi T, Yasunaga H, Miyashiro I. Comparison of overall mortality between hysterectomy and concurrent chemoradiotherapy for the primary treatment of localized or regionally extended cervical cancer. Annals of clinical epidemiology. In press.
Morishima T SA, Nakata K, Miyashiro I. Geriatric assessment domains to predict overall survival in older cancer patients: an analysis of functional status, comorbidities, and nutritional status as prognostic factors. Cancer Medicine. In press.
Miyamoto Y, Ohbe H, Ishimaru M, Matsui H, Fushimi K, Yasunaga H. The effect of carbazochrome sodium sulfonate in patients with colonic diverticular bleeding: propensity score matching analyses using a Nationwide inpatient database. Inter Med. 2020;59(15):1789–94.
International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987–94.
Gallamini A, Stelitano C, Calvi R, Bellei M, Mattei D, Vitolo U, et al. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103(7):2474–9.
Mourad N, Mounier N, Brière J, Raffoux E, Delmer A, Feller A, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111(9):4463–70.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
Ellin F, Landstrom J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood. 2014;124(10):1570–7.
Cederleuf H, Bjerregård Pedersen M, Jerkeman M, Relander T, d’Amore F, Ellin F. The addition of etoposide to CHOP is associated with improved outcome in ALK+ adult anaplastic large cell lymphoma: a Nordic Lymphoma Group study. Br J Haematol. 2017;178(5):739–46.
Janikova A, Chloupkova R, Campr V, Klener P, Hamouzova J, Belada D, et al. First-line therapy for T cell lymphomas: a retrospective population-based analysis of 906 T cell lymphoma patients. Ann Hematol. 2019;98(8):1961–72.
Shichijo T. Hematopoietic stem cell transplantation for T-cell lymphoma. Adv Cell Gene Ther. 2018;1(1):e6.
Vose J, Armitage J, Weisenburger D, International TCLP. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–30.
Horwitz S, O’Connor OA, Pro B, Illidge T, Fanale M, Advani R, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229–40.
Funding
This research was partially supported by a Health, Labor, and Welfare Sciences Research Grant from the Ministry of Health, Labor, and Welfare of Japan (H30-Gantaisaku-ippan-009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict of interest to declare.
Ethical approval
The study was approved by the institutional review board of the Osaka International Cancer Institute in Osaka, Japan (No. 19088).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kida, S., Fuji, S., Morishima, T. et al. Comparison of CHOP with THP-COP for peripheral T-cell lymphoma-not otherwise specified and angioimmunoblastic T-cell lymphoma: a retrospective analysis using data from the population-based Osaka Cancer Registry. Int J Hematol 114, 246–251 (2021). https://doi.org/10.1007/s12185-021-03150-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03150-6