Abstract
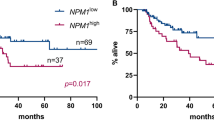
Minimal residual disease (MRD) monitoring by quantitative real-time reverse transcription PCR (qRT-PCR) is the standard of care in Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph-positive ALL). We evaluated the impact of MRD status at hematopoietic cell transplantation (HCT) on relapse, as measured by a unified protocol at a central laboratory. Only patients with Ph-positive ALL who had minor transcripts (e1a2) and who underwent allogeneic HCT in first complete remission between 2008 and 2017 were included. First, patients with negative-MRD (n = 196) and positive-MRD (n = 61) at HCT were analyzed. As expected, MRD positivity at HCT was significantly associated with an increased risk of hematological relapse (hazard ratio [HR], 2.91; 95% CI 1.67–5.08; P < 0.001) in the multivariate analysis. Next, patients with positive-MRD were divided into low-MRD (n = 39) and high-MRD (n = 22) groups. In the multivariate analysis, high-MRD at HCT was not significantly associated with an increased risk of hematological relapse compared to the low-MRD group (HR 1.10; 95% CI 0.54–2.83; P = 0.620). These results indicate that the therapeutic decisions should be made based on MRD positivity, rather than on the MRD level, at HCT.


Similar content being viewed by others
References
van Dongen JJ, van der Velden VH, Bruggemann M, Orfao A. Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood. 2015;125:3996–4009.
Bassan R, Bruggemann M, Radcliffe HS, Hartfield E, Kreuzbauer G, Wetten S. A systematic literature review and meta-analysis of minimal residual disease as a prognostic indicator in adult B-cell acute lymphoblastic leukemia. Haematologica. 2019;104:2028–39.
Mizuta S, Matsuo K, Nishiwaki S, Imai K, Kanamori H, Ohashi K, et al. Pretransplant administration of imatinib for allo-HSCT in patients with BCR-ABL-positive acute lymphoblastic leukemia. Blood. 2014;123:2325–32.
Nishiwaki S, Imai K, Mizuta S, Kanamori H, Ohashi K, Fukuda T, et al. Impact of MRD and TKI on allogeneic hematopoietic cell transplantation for Ph+ALL: a study from the adult ALL WG of the JSHCT. Bone Marrow Transplant. 2016;51:43–50.
Lussana F, Intermesoli T, Gianni F, Boschini C, Masciulli A, Spinelli O, et al. Achieving molecular remission before allogeneic stem cell transplantation in adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: impact on relapse and long-term outcome. Biol Blood Marrow Transplant. 2016;22:1983–7.
Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37.
Pfeifer H, Cazzaniga G, van der Velden VHJ, Cayuela JM, Schafer B, Spinelli O, et al. Standardisation and consensus guidelines for minimal residual disease assessment in Philadelphia-positive acute lymphoblastic leukemia (Ph + ALL) by real-time quantitative reverse transcriptase PCR of e1a2 BCR-ABL1. Leukemia. 2019;33:1910–22.
Ravandi F, O’Brien S, Thomas D, Faderl S, Jones D, Garris R, et al. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116:2070–7.
Jabbour E, Kantarjian H, Ravandi F, Thomas D, Huang X, Faderl S, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a single-centre, phase 2 study. Lancet Oncol. 2015;16:1547–55.
Short NJ, Jabbour E, Sasaki K, Patel K, O’Brien SM, Cortes JE, et al. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2016;128:504–7.
Akahoshi Y, Nishiwaki S, Arai Y, Harada K, Najima Y, Kanda Y, et al. Reduced-intensity conditioning is a reasonable alternative for Philadelphia chromosome-positive acute lymphoblastic leukemia among elderly patients who have achieved negative minimal residual disease: a report from the Adult Acute Lymphoblastic Leukemia Working Group of the JSHCT. Bone Marrow Transplant. 2020;55:1317–25.
Atsuta Y. Introduction of Transplant Registry Unified Management Program 2 (TRUMP2): scripts for TRUMP data analyses, part I (variables other than HLA-related data). Int J Hematol. 2016;103:3–10.
Kanda J. Scripts for TRUMP data analyses. Part II (HLA-related data): statistical analyses specific for hematopoietic stem cell transplantation. Int J Hematol. 2016;103:11–9.
Yokota H, Tsuno NH, Tanaka Y, Fukui T, Kitamura K, Hirai H, et al. Quantification of minimal residual disease in patients with e1a2 BCR-ABL-positive acute lymphoblastic leukemia using a real-time RT-PCR assay. Leukemia. 2002;16:1167–75.
Foroni L, Wilson G, Gerrard G, Mason J, Grimwade D, White HE, et al. Guidelines for the measurement of BCR-ABL1 transcripts in chronic myeloid leukaemia. Br J Haematol. 2011;153:179–90.
Wang J, Jiang Q, Xu LP, Zhang XH, Chen H, Qin YZ, et al. Allogeneic stem cell transplantation versus tyrosine kinase inhibitors combined with chemotherapy in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2018;24:741–50.
Akahoshi Y, Mizuta S, Shimizu H, Uchida N, Fukuda T, Kanamori H, et al. Additional cytogenetic abnormalities with Philadelphia chromosome-positive acute lymphoblastic leukemia on allogeneic stem cell transplantation in the tyrosine kinase inhibitor era. Biol Blood Marrow Transplant. 2018;24:2009–16.
Mohty M, Labopin M, Volin L, Gratwohl A, Socie G, Esteve J, et al. Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2010;116:4439–43.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–9.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Pagani IS, Spinelli O, Mattarucchi E, Pirrone C, Pigni D, Amelotti E, et al. Genomic quantitative real-time PCR proves residual disease positivity in more than 30% samples with negative mRNA-based qRT-PCR in Chronic Myeloid Leukemia. Oncoscience. 2014;1:510–21.
Bader P, Salzmann-Manrique E, Balduzzi A, Dalle JH, Woolfrey AE, Bar M, et al. More precisely defining risk peri-HCT in pediatric ALL: pre- vs post-MRD measures, serial positivity, and risk modeling. Blood Adv. 2019;3:3393–405.
Pagani IS, Dang P, Kommers IO, Goyne JM, Nicola M, Saunders VA, et al. BCR-ABL1 genomic DNA PCR response kinetics during first-line imatinib treatment of chronic myeloid leukemia. Haematologica. 2018;103:2026–32.
Akahoshi Y, Igarashi A, Fukuda T, Uchida N, Tanaka M, Ozawa Y, et al. Impact of graft-versus-host disease and graft-versus-leukemia effect based on minimal residual disease in Philadelphia chromosome-positive acute lymphoblastic leukemia. Br J Haematol. 2020;190:84–92.
Beillard E, Pallisgaard N, van der Velden VH, Bi W, Dee R, van der Schoot E, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using “real-time” quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR)—a Europe against cancer program. Leukemia. 2003;17:2474–86.
Sun Y, Li Y, Luo D, Liao DJ. Pseudogenes as weaknesses of ACTB (Actb) and GAPDH (Gapdh) used as reference genes in reverse transcription and polymerase chain reactions. PLoS ONE. 2012;7:e41659.
Towatari M, Yanada M, Usui N, Takeuchi J, Sugiura I, Takeuchi M, et al. Combination of intensive chemotherapy and imatinib can rapidly induce high-quality complete remission for a majority of patients with newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia. Blood. 2004;104:3507–12.
Yanada M, Takeuchi J, Sugiura I, Akiyama H, Usui N, Yagasaki F, et al. High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol. 2006;24:460–6.
Mizuta S, Matsuo K, Yagasaki F, Yujiri T, Hatta Y, Kimura Y, et al. Pre-transplant imatinib-based therapy improves the outcome of allogeneic hematopoietic stem cell transplantation for BCR-ABL-positive acute lymphoblastic leukemia. Leukemia. 2011;25:41–7.
Fujisawa S, Mizuta S, Akiyama H, Ueda Y, Aoyama Y, Hatta Y, et al. Phase II study of imatinib-based chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia. Am J Hematol. 2017;92:367–74.
Akahoshi Y, Nishiwaki S, Mizuta S, Ohashi K, Uchida N, Tanaka M, et al. Tyrosine kinase inhibitor prophylaxis after transplant for Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer Sci. 2019;110:3255–66.
Giebel S, Czyz A, Ottmann O, Baron F, Brissot E, Ciceri F, et al. Use of tyrosine kinase inhibitors to prevent relapse after allogeneic hematopoietic stem cell transplantation for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: a position statement of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Cancer. 2016;122:2941–51.
Acknowledgements
The authors greatly appreciate the contributions of many physicians and data managers throughout the JSHCT, the Japan Marrow Donor Program (JMDP), and the Japan Cord Blood Bank Network (JCBBN), who made this analysis possible. We would also like to thank the members of the Transplant Registry Unified Management committees at JSHCT, JMDP, and JCBBN for their dedicated management of data. We are grateful to the team of technicians in the RNA Analysis Section, Genetic and Pathology Department, SRL, Inc. for their excellent work. Y. Akahoshi is a Research Fellow of Japan Society of the Promotion of Science (JSPS) and this work was supported by JSPS KAKENHI Grant Number JP20J10298.
Author information
Authors and Affiliations
Contributions
Y. Akahoshi designed the study, analyzed the data, and wrote the manuscript; Y. Arai, SN, SM, and SK reviewed and revised the manuscript; AM, NU, YK, HS, ST, TF, SF, TA, JT, and Y. Atsuta collected the patient data; all authors contributed to the writing of the report and approved the final version of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests relevant to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
On behalf of the Adult Acute Lymphoblastic Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation.
About this article
Cite this article
Akahoshi, Y., Arai, Y., Nishiwaki, S. et al. Minimal residual disease (MRD) positivity at allogeneic hematopoietic cell transplantation, not the quantity of MRD, is a risk factor for relapse of Philadelphia chromosome-positive acute lymphoblastic leukemia. Int J Hematol 113, 832–839 (2021). https://doi.org/10.1007/s12185-021-03094-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03094-x