Abstract
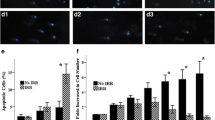
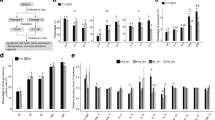
Two gray γ-irradiation is a widely employed basic module for total body irradiation (TBI) in allogeneic hematopoietic cell transplantation (HCT). The effects of γ-irradiation on hematopoietic and immune cells have been well investigated, but its effects on the bone marrow microenvironment (BMM) are unknown. Given the crucial contribution of mesenchymal/stromal stem cells (MSCs) in the BMM to hematopoiesis and osteogenesis, we investigated whether γ-irradiation affects the hallmark characteristics of human bone marrow-derived MSCs (BM-MSCs). Expansion of 2 Gy γ-irradiated BM-MSCs was delayed but eventually recovered. Colony formation and osteogenic, adipogenic, and chondrogenic differentiation capabilities of these cells were extensively suppressed. Irradiation of BM-MSCs did not affect the expansion of CD34 + hematopoietic stem and progenitor cells or production of CD11b + mature myeloid cells in co-cultures. However, it reduced production of CD19 + B-cells, as well as expression of CXCL12 and interleukin-7, which are essential for B-cell lymphopoiesis, in 2 Gy γ-irradiated BM-MSCs. Collectively, colony formation, osteogenic differentiation, and B-cell lymphopoiesis-supportive capabilities of γ-irradiated BM-MSCs were reduced. These effects may predispose survivors receiving HCT with TBI to defective bone formation and a perturbed humoral immune response.



Similar content being viewed by others
References
Thomas ED, Clift RA, Hersman J, Sanders JE, Stewart P, Buckner CD, et al. Marrow transplantation for acute nonlymphoblastic leukemic in first remission using fractionated or single-dose irradiation. Int J Radiat Oncol Biol Phys. 1982;8:817–21. https://doi.org/10.1016/0360-3016(82)90083-9.
Deeg HJ, Sullivan KM, Buckner CD, Storb R, Appelbaum FR, Clift RA, et al. Marrow transplantation for acute nonlymphoblastic leukemia in first remission: toxicity and long-term follow-up of patients conditioned with single dose or fractionated total body irradiation. Bone Marrow Transplant. 1986;1:151–7.
Thomas ED, Buckner CD, Banaji M, Clift RA, Fefer A, Flournoy N, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. 1977;49:511–33.
Storb R, Gyurkocza B, Storer BE, Sorror ML, Blume K, Niederwieser D, et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2013;31:1530–8. https://doi.org/10.1200/jco.2012.45.0247.
Majhail NS, Rizzo JD. Surviving the cure: long term followup of hematopoietic cell transplant recipients. Bone Marrow Transplant. 2013;48:1145–51. https://doi.org/10.1038/bmt.2012.258.
Bhatia S, Francisco L, Carter A, Sun CL, Baker KS, Gurney JG, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–92. https://doi.org/10.1182/blood-2007-03-082933.
Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–34. https://doi.org/10.1038/nature09262.
Fekete N, Erle A, Amann EM, Fürst D, Rojewski MT, Langonné A, et al. Effect of high-dose irradiation on human bone-marrow-derived mesenchymal stromal cells. Tissue Eng Part C Methods. 2015;21:112–22. https://doi.org/10.1089/ten.tec.2013.0766.
Fujishiro A, Miura Y, Iwasa M, Fujii S, Sugino N, Andoh A, et al. Effects of acute exposure to low-dose radiation on the characteristics of human bone marrow mesenchymal stromal/stem cells. Inflamm Regen. 2017;37:19. https://doi.org/10.1186/s41232-017-0049-2.
Pelszynski MM, Moroff G, Luban NL, Taylor BJ, Quinones RR. Effect of gamma irradiation of red blood cell units on T-cell inactivation as assessed by limiting dilution analysis: implications for preventing transfusion-associated graft-versus-host disease. Blood. 1994;83:1683–9.
Preciado S, Muntión S, Rico A, Pérez-Romasanta LA, Ramos TL, Ortega R, et al. Mesenchymal stromal cell irradiation interferes with the adipogenic/osteogenic differentiation balance and improves their hematopoietic-supporting ability. Biol Blood Marrow Transplant. 2018;24:443–51. https://doi.org/10.1016/j.bbmt.2017.11.007.
Iwasa M, Miura Y, Fujishiro A, Fujii S, Sugino N, Yoshioka S, et al. Bortezomib interferes with adhesion of B cell precursor acute lymphoblastic leukemia cells through SPARC up-regulation in human bone marrow mesenchymal stromal/stem cells. Int J Hematol. 2017;105:587–97. https://doi.org/10.1007/s12185-016-2169-x.
Fujii S, Miura Y, Fujishiro A, Shindo T, Shimazu Y, Hirai H, et al. Graft-versus-host disease amelioration by human bone marrow mesenchymal stromal/stem cell-derived extracellular vesicles is associated with peripheral preservation of naive T cell populations. Stem Cells. 2018;36:434–45. https://doi.org/10.1002/stem.2759.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. https://doi.org/10.1080/14653240600855905.
Sugino N, Miura Y, Yao H, Iwasa M, Fujishiro A, Fujii S, et al. Early osteoinductive human bone marrow mesenchymal stromal/stem cells support an enhanced hematopoietic cell expansion with altered chemotaxis- and adhesion-related gene expression profiles. Biochem Biophys Res Commun. 2016;469:823–9. https://doi.org/10.1016/j.bbrc.2015.12.061.
Yao H, Miura Y, Yoshioka S, Miura M, Hayashi Y, Tamura A, et al. Parathyroid hormone enhances hematopoietic expansion via upregulation of cadherin-11 in bone marrow mesenchymal stromal cells. Stem Cells. 2014;32:2245–55. https://doi.org/10.1002/stem.1701.
Ichii M, Oritani K, Yokota T, Schultz DC, Holter JL, Kanakura Y, et al. Stromal cell-free conditions favorable for human B lymphopoiesis in culture. J Immunol Methods. 2010;359:47–55. https://doi.org/10.1016/j.jim.2010.06.002.
Alessio N, Del Gaudio S, Capasso S, Di Bernardo G, Cappabianca S, Cipollaro M, et al. Low-dose radiation induced senescence of human mesenchymal stromal cells and impaired the autophagy process. Oncotarget. 2015;6:8155–66. https://doi.org/10.18632/oncotarget.2692.
Osipov AN, Pustovalova M, Grekhova A, Eremin P, Vorobyova N, Pulin A, et al. Low doses of X-rays induce prolonged and ATM-independent persistence of γH2AX foci in human gingival mesenchymal stem cells. Oncotarget. 2015;6:27275–87. https://doi.org/10.18632/oncotarget.4739.
Laurent A, Blasi F. Differential DNA damage signaling and apoptotic threshold correlate with mouse epiblast-specific hypersensitivity to radiation. Development. 2015;142:3675–85. https://doi.org/10.1242/dev.125708.
Tubbs A, Nussenzweig A. Endogenous DNA damage as a source of genomic instability in cancer. Cell. 2017;168:644–56. https://doi.org/10.1016/j.cell.2017.01.002.
Lomax M, Folkes L, O’Neill P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin Oncol (R Coll Radiol). 2013;25:578–85. https://doi.org/10.1016/j.cell.2017.01.002.
Vilenchik MM, Knudson AG. Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proc Natl Acad Sci USA. 2003;100:12871–6. https://doi.org/10.1073/pnas.2135498100.
Dias S, Silva H Jr, Cumano A, Vieira P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J Exp Med. 2005;201:971–9. https://doi.org/10.1084/jem.20042393.
Egawa T, Kawabata K, Kawamoto H, Amada K, Okamoto R, Fujii N, et al. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity. 2001;15:323–34. https://doi.org/10.1016/s1074-7613(01)00185-6.
Zhou H, Choong P, McCarthy R, Chou S, Martin T, Ng KW. In situ hybridization to show sequential expression of osteoblast gene markers during bone formation in vivo. J Bone Miner Res. 1994;9:1489–99. https://doi.org/10.1002/jbmr.5650090922.
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–64. https://doi.org/10.1016/s0092-8674(00)80258-5.
Golub EE, Boesze-Battaglia K. The role of alkaline phosphatase in mineralization. Curr Opin Orthop. 2007;18:444–8. https://doi.org/10.1097/bco.0b013e3282630851.
Cutler C, Ballen K. Reduced-intensity conditioning and umbilical cord blood transplantation in adults. Bone Marrow Transplant. 2009;44:667–71. https://doi.org/10.1038/bmt.2009.283.
Takahashi S, Ooi J, Tomonari A, Konuma T, Tsukada N, Oiwa-Monna M, et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood. 2007;109:1322–30. https://doi.org/10.1182/blood-2006-04-020172.
Muller-Sieburg CE, Cho RH, Karlsson L, Huang JF, Sieburg HB. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood. 2004;103:4111–8. https://doi.org/10.1182/blood-2003-10-3448.
Velardi E, Tsai JJ, van den Brink MRM. T cell regeneration after immunological injury. Nat Rev Immunol. 2020. https://doi.org/10.1038/s41577-020-00457-z.
Krampera M, Sartoris S, Liotta F, Pasini A, Angeli R, Cosmi L, et al. Immune regulation by mesenchymal stem cells derived from adult spleen and thymus. Stem Cells Dev. 2007;16:797–810. https://doi.org/10.1089/scd.2007.0024.
Patenaude J, Perreault C. Thymic mesenchymal cells have a distinct transcriptomic profile. J Immunol. 2016;196:4760–70. https://doi.org/10.4049/jimmunol.1502499.
Suniara RK, Jenkinson EJ, Owen JJ. An essential role for thymic mesenchyme in early T cell development. J Exp Med. 2000;191:1051–6. https://doi.org/10.1084/jem.191.6.1051.
Acknowledgements
We thank Ms. Yoko Nakagawa for excellent technical assistance.
Funding
This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology in Japan (#18K08323 to Y.M., #19K17856 to S.F.) and by the Japanese Society of Hematology Research Grant (Y.M. and S.F.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Ichinohe has received research funding from FUJIFILM Wako Chemicals, Repertoire Genesis Inc., and Takara Bio Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Iwasa, M., Fujii, S., Fujishiro, A. et al. Impact of 2 Gy γ-irradiation on the hallmark characteristics of human bone marrow-derived MSCs. Int J Hematol 113, 703–711 (2021). https://doi.org/10.1007/s12185-020-03072-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-020-03072-9