Abstract
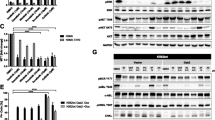
Nilotinib is a substrate of the breast cancer resistance protein (BCRP), which is a drug efflux transporter encoded by ABCG2 and regulates the pharmacokinetics of its substrates. We investigated the interaction between nilotinib and BCRP in chronic myeloid leukemia (CML) cells. An imatinib-resistant K562 cell line (K562/IM-R) treated with nilotinib was analyzed for BCRP expression, proliferation, apoptosis, and intracellular nilotinib concentration. K562/IM-R cells cultured with tyrosine kinase inhibitors (TKIs) showed an increased cell count and retained viability, whereas the growth of parental K562 cells was severely inhibited, suggesting that BCRP is involved in developing resistance to TKIs. Nilotinib-treated K562/IM-R cells showed a reduction in apoptosis; however, febuxostat pretreatment resulted in increased apoptosis. The intracellular concentration of nilotinib in K562/IM-R cells was significantly reduced compared to that in parental K562 cells, and febuxostat-pretreated K562/IM-R cells showed an increased intracellular nilotinib level compared to cells without pretreatment. The reduction in nilotinib levels caused by BCRP in CML cells might play a crucial role in resistance to TKIs. Moreover, febuxostat, as a BCRP inhibitor, could enhance nilotinib sensitivity, and combination therapy with nilotinib and febuxostat may represent a promising strategy for treatment of CML.


Similar content being viewed by others
References
Soverini S, Hochhaus A, Nicolini FE, Gruber F, Lange T, Saglio G, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitor: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118:1208–15.
Loscocco F, Visani G, Galimberti S, Curti A, Isidori A. BCR-ABL independent mechanisms of resistance in chronic myeloid leukemia. Front Oncol. 2019;9:939.
Hekmatshoar Y, Ozkan T, Gunes BA, Bozkurt S, Karadag A, Karabay AZ, et al. Characterization of imatinib-resistant K562 cell line displaying resistance mechanisms. Cell Mol Biol. 2018;64:23–30.
Szakács G, Váradi A, Özvegy-Laczka C, Sarkadi B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox). Drug Discov Today. 2008;13:379–93.
Noguchi K, Katayama K, Mitsuhashi J, Sugimoto Y. Functions of the breast cancer resistance protein (BCRP/ABCG2) in chemotherapy. Adv Drug Deliv Rev. 2009;61:26–33.
Jordanides NE, Jorgensen HG, Holyoake TL, Mountford JC. Functional ABCG2 is overexpressed on primary CML CD34+ cells and is inhibited by imatinib mesylate. Blood. 2009;108:1370–3.
Brendel C, Scharenberg C, Dohse M, Robey RW, Bates SE, Shukla S, et al. Imatinib mesylate and nilotinib (AMN107) exhibit high-affinity interaction with ABCG2 on primitive hematopoietic stem cells. Leukemia. 2007;21:1267–75.
Hegedus C, Ozvegy-Laczka C, Apáti A, Magócsi M, Német K, Orfi L, et al. Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological properties. Br J Pharmacol. 2009;158:1153–64.
Maekawa K, Itoda M, Sai K, Saito Y, Kaniwa N, Shirao K, et al. Genetic variation and haplotype structure of the ABC transporter gene ABCG2 in a Japanese population. Drug Metab Pharmacokinet. 2006;21:109–21.
Imai Y, Nakane M, Kage K, Tsukahara S, Ishikawa E, Tsuruo T, et al. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Ther. 2002;1:611–6.
Noguchi S, Nakaseko C, Nishiwaki K, Ogasawara H, Ohishi K, et al. Switching to nilotinib is associated with deeper molecular responses in chronic myeloid leukemia chronic phase with major molecular responses to imatinib: STAT1 trial in Japan. Int J Hematol. 2018;108:176–83.
Miyata H, Takada T, Toyoda Y, Matsuo H, Ichida K, Suzuki H. Identification of febuxostat as a new strong ABCG2 inhibitor: potential applications and risks in clinical situations. Front Pharmacol. 2016;7:518.
Miura M, Takahashi N, Sawada K. High-performance liquid chromatography with solid-phase extraction for the quantitative determination of nilotinib in human plasma. Biomed Chromatogr. 2010;24:789–93.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Allen JD, Loevezijn AV, Lakhai JM, Valk MVD, Tellingen OV, Reid G, et al. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of Fumitremorgin C. Mol Cancer Ther. 2002;1:417–25.
Weidner LD, Zoghbi SS, Lu S, Shukla S, Ambudkar SV, Pike VW, et al. The inhibitor Ko143 is not specific for ABCG2. J Pharmacol Exp Ther. 2015;354:384–93.
Hyafil F, Vergely C, Vignaud PD, Grand-Perret T. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 1993;53:4595–602.
Mahon FX, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–35.
Takahashi N, Tauchi N, Kitamura K, Miyamura K, Saburi Y, Hatta Y, et al. Deeper molecular response is a predictive factor for treatment-free remission after imatinib discontinuation in patients with chronic phase chronic myeloid leukemia: the JALSG-STIM213 study. Int J Hematol. 2018;107:185–93.
Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–34.
Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–12.
Rinaldetti S, Pfirrmann M, Manz K, Guilhot J, Dietz C, Panagiotidis P, et al. Effect of ABCG2, OCT1, and ABCB1 (MDR1) gene expression on treatment-free remission in a EURO-SKI subtrial. Clin Lymphoma Myeloma Leuk. 2018;18:266–71.
Acknowledgements
We would like to thank Editage (https://www.editage.com) for English editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Naoto Takahashi reports that he has received Grants and speaker’s fees from Novartis Pharmaceuticals, speaker’s fees from Bristol Myers Squibb, Grants and speaker’s fees from Pfizer, Grants and speaker’s fees from Otsuka Pharmaceutical, Grants from Kyowa Hakko Kirin, Grants from Astellas Pharma, Grants from Chugai Pharmaceutical, Grants from Asahi Kasei Pharma, Grants from Ono Pharmaceutical, and Grants from Eisai Pharmaceuticals, outside of the submitted work. The remaining authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ito, F., Miura, M., Fujioka, Y. et al. The BCRP inhibitor febuxostat enhances the effect of nilotinib by regulation of intracellular concentration. Int J Hematol 113, 100–105 (2021). https://doi.org/10.1007/s12185-020-03000-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-020-03000-x