Abstract
Purpose of Review
This review summarizes the current state of knowledge regarding the pharmacogenetics of antiplatelet and anticoagulant medications. Barriers to clinical implementation and important research gaps in the field are also discussed.
Recent Findings
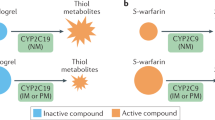
Two large prospective randomized clinical trials have been conducted in the percutaneous coronary intervention (PCI) population showing that CYP2C19-guided antiplatelet prescribing reduces ischemic and bleeding events. Clinical implementation barriers for widespread adoption included availability of a rapid turnaround CYP2C19 test and endorsement of testing by cardiology guidelines. Data are also emerging on the benefits of CYP2C19 testing for neurovascular indications. Three large prospective trials have been conducted on genetically guided warfarin dosing; the two studies that had positive outcomes were performed in mainly European populations. The third trial showed harm in participants of African ethnicity, limiting the clinical translation of warfarin pharmacogenetics.
Summary
Tremendous progress has been made in the discovery and clinical implementation of pharmacogenetics for personalizing antiplatelet and anticoagulant medications. Prospective pharmacogenetic testing is feasible, but important implementation barriers remain for widespread clinical adoption. The identification of variants influencing drug response in all ethnic groups is vital to ensure equity and not widen health disparities in the application of pharmacogenetics.
Similar content being viewed by others
References
Relling MV, Evans WE. Pharmacogenomics in the clinic. Nature. 2015;526(7573):343–50.
Nussbaum RL. Thompson & Thompson genetics in medicine. In: McInnes RR, Willard HF, editors. 8th ed.
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200–5.
Gandhi TK, Weingart SN, Borus J, Seger AC, Peterson J, Burdick E, et al. Adverse drug events in ambulatory care. N Engl J Med. 2003;348(16):1556–64.
Spear BB, Heath-Chiozzi M, Huff J. Clinical application of pharmacogenetics. Trends Mol Med. 2001;7(5):201–4.
Watanabe JH, McInnis T, Hirsch JD. Cost of prescription drug-related morbidity and mortality. Ann Pharmacother. 2018;52(9):829–37.
Bush WS, Crosslin DR, Obeng AO, Wallace J, Almoguera B, Basford MA, et al. Genetic variation among 82 pharmacogenes: the PGRN-Seq data from the eMERGE Network. Clin Pharmacol Ther. 2016;100(2):160–9.
Van Driest SL, Shi Y, Bowton EA, Schildcrout JS, Peterson JF, Pulley J, et al. Clinically actionable genotypes among 10,000 patients with preemptive pharmacogenomic testing. Clin Pharmacol Ther. 2014;95(4):423–31.
Chanfreau-Coffinier C, Hull LE, Lynch JA, DuVall SL, Damrauer SM, Cunningham FE, et al. Projected prevalence of actionable pharmacogenetic variants and level a drugs prescribed among US veterans health administration pharmacy users. JAMA Netw Open. 2019;2(6):e195345.
McInnes G, Lavertu A, Sangkuhl K, Klein TE, Whirl-Carrillo M, Altman RB. Pharmacogenetics at scale: an analysis of the UK Biobank. Clin Pharmacol Ther. 2021;109(6):1528–37.
Drozda K, Pacanowski MA, Grimstein C, Zineh I. Pharmacogenetic labeling of FDA-approved drugs: a regulatory retrospective. JACC Basic Transl Sci. 2018;3(4):545–9.
CPIC: Clinical pharmacogenetics implementation consortium. https://cpicpgx.org/ (accessed March 8, 2016).
Scott SA, Sangkuhl K, Gardner EE, Stein CM, Hulot JS, Johnson JA, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450–2C19 (CYP2C19) genotype and clopidogrel therapy. Clin Pharmacol Ther. 2011;90(2):328–32.
Johnson JA, Gong L, Whirl-Carrillo M, Gage BF, Scott SA, Stein CM, et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C9 and VKORC1 Genotypes and Warfarin Dosing. Clin Pharmacol Ther. 2011;90(4):625–9.
Lee CR, Luzum JA, Sangkuhl K, Gammal RS, Sabatine MS, Stein CM, et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2C19 Genotype and Clopidogrel Therapy: 2022 Update. Clin Pharmacol Ther. 2022;112(5):959–67.
Johnson JA, Caudle KE, Gong L, Whirl-Carrillo M, Stein CM, Scott SA, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Pharmacogenetics-Guided Warfarin Dosing: 2017 Update. Clin Pharmacol Ther. 2017;102(3):397–404.
Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134(10):e123–55.
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58(24):e44–122.
Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015;12(1):30–47.
Brandt JT, Close SL, Iturria SJ, Payne CD, Farid NA, Ernest CS 2nd, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost JTH. 2007;5(12):2429–36.
Gurbel PA, Bergmeijer TO, Tantry US, ten Berg JM, Angiolillo DJ, James S, et al. The effect of CYP2C19 gene polymorphisms on the pharmacokinetics and pharmacodynamics of prasugrel 5-mg, prasugrel 10-mg and clopidogrel 75-mg in patients with coronary artery disease. Thromb Haemost. 2014;112(3):589–97.
Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–15.
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–57.
Dayoub EJ, Seigerman M, Tuteja S, Kobayashi T, Kolansky DM, Giri J, et al. Trends in platelet adenosine diphosphate P2Y12 receptor inhibitor use and adherence among antiplatelet-naive patients after percutaneous coronary intervention, 2008–2016. JAMA Intern Med. 2018;178(7):943–50.
Dayoub EJ, Nathan AS, Khatana SAM, Seigerman M, Tuteja S, Kobayashi T, et al. Use of prasugrel and ticagrelor in stable ischemic heart disease after percutaneous coronary intervention, 2009–2016. Circ Cardiovasc Interv. 2019;12(1):e007434.
Karve AM, Seth M, Sharma M, LaLonde T, Dixon S, Wohns D, et al. Contemporary use of ticagrelor in interventional practice (from Blue Cross Blue Shield of Michigan Cardiovascular Consortium). Am J Cardiol. 2015;115(11):1502–6.
Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38(1):92–9.
Sibbing D, Aradi D, Alexopoulos D, ten Berg J, Bhatt DL, Bonello L, et al. Updated Expert Consensus Statement on Platelet Function and Genetic Testing for Guiding P2Y12 Receptor Inhibitor Treatment in Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2019;12(16):1521–37.
Sibbing D, Koch W, Gebhard D, Schuster T, Braun S, Stegherr J, et al. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation. 2010;121(4):512–8.
Pereira NL, Rihal CS, So DYF, Rosenberg Y, Lennon RJ, Mathew V, et al. Clopidogrel pharmacogenetics. Circ Cardiovasc Interv. 2019;12(4):e007811.
Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302(8):849–57.
Lewis JP, Backman JD, Reny JL, Bergmeijer TO, Mitchell BD, Ritchie MD, et al. Pharmacogenomic polygenic response score predicts ischemic events and cardiovascular mortality in clopidogrel-treated patients. Eur Heart J Cardiovasc Pharmacother. 2020;6(4):203–10.
Lewis JP, Stephens SH, Horenstein RB, O’Connell JR, Ryan K, Peer CJ, et al. The CYP2C19*17 variant is not independently associated with clopidogrel response. J Thromb Haemost JTH. 2013;11(9):1640–6.
Lee CR, Thomas CD, Beitelshees AL, Tuteja S, Empey PE, Lee JC, et al. Impact of the CYP2C19*17 allele on outcomes in patients receiving genotype-guided antiplatelet therapy after percutaneous coronary intervention. Clin Pharmacol Ther. 2021;109(3):705–15.
Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304(16):1821–30.
Cavallari LH, Lee CR, Beitelshees AL, Cooper-DeHoff RM, Duarte JD, Voora D, et al. Multisite investigation of outcomes with implementation of CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. JACC Cardiovasc Interve. 2017.
Shen DL, Wang B, Bai J, Han Q, Liu C, Huang XH, et al. Clinical value of CYP2C19 genetic testing for guiding the antiplatelet therapy in a Chinese population. J Cardiovasc Pharmacol. 2016;67(3):232–6.
Hulot JS, Chevalier B, Belle L, Cayla G, Khalife K, Funck F, et al. Routine CYP2C19 genotyping to adjust thienopyridine treatment after primary PCI for STEMI: results of the GIANT study. JACC Cardiovasc Interv. 2020;13(5):621–30.
Beitelshees AL, Thomas CD, Empey PE, Stouffer GA, Angiolillo DJ, Franchi F, et al. CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention in diverse clinical settings. J Am Heart Assoc. 2022;11(4):e024159.
Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van’t Hof AWJ, van der Harst P, et al. A genotype-guided strategy for oral P2Y12 inhibitors in primary PCI. N Engl J Med. 2019;381(17):1621–31.
Pereira NL, Farkouh ME, So D, Lennon R, Geller N, Mathew V, et al. Effect of genotype-guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: the TAILOR-PCI randomized clinical trial. JAMA. 2020;324(8):761–71.
Notarangelo FM, Maglietta G, Bevilacqua P, Cereda M, Merlini PA, Villani GQ, et al. Pharmacogenomic approach to selecting antiplatelet therapy in patients with acute coronary syndromes: the PHARMCLO trial. J Am Coll Cardiol. 2018;71(17):1869–77.
Zhang Y, Zhao Y, Pang M, Wu Y, Zhuang K, Zhang H, et al. High-dose clopidogrel versus ticagrelor for treatment of acute coronary syndromes after percutaneous coronary intervention in CYP2C19 intermediate or poor metabolizers: a prospective, randomized, open-label, single-centre trial. Acta Cardiol. 2016;71(3):309–16.
Ogawa H, Isshiki T, Kimura T, Yokoi H, Nanto S, Takayama M, et al. Effects of CYP2C19 allelic variants on inhibition of platelet aggregation and major adverse cardiovascular events in Japanese patients with acute coronary syndrome: The PRASFIT-ACS study. J Cardiol. 2016;68(1):29–36.
Tuteja S, Glick H, Matthai W, Nachamkin I, Nathan A, Monono K, et al. Prospective CYP2C19 genotyping to guide antiplatelet therapy following percutaneous coronary intervention: a pragmatic randomized clinical trial. Circ Genom Precis Med. 2020;13(1):e002640.
Sánchez-Ramos J, Dávila-Fajardo CL, Toledo Frías P, Díaz Villamarín X, Martínez-González LJ, Martínez Huertas S, et al. Results of genotype-guided antiplatelet therapy in patients who undergone percutaneous coronary intervention with stent. Int J Cardiol. 2016;225:289–95.
Lee CR, Sriramoju VB, Cervantes A, Howell LA, Varunok N, Madan S, et al. Clinical outcomes and sustainability of using CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. Circ Genom Precis Med. 2018;11(4):e002069.
Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369(1):11–9.
Pan Y, Chen W, Xu Y, Yi X, Han Y, Yang Q, et al. Genetic polymorphisms and clopidogrel efficacy for acute ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Circulation. 2017;135(1):21–33.
Wang Y, Meng X, Wang A, Xie X, Pan Y, Johnston SC, et al. Ticagrelor versus clopidogrel in CYP2C19 loss-of-function carriers with stroke or TIA. N Engl J Med. 2021;385(27):2520–30.
Holmes DR Jr, Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA Clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association. Circulation. 2010;122(5):537–57.
US Food and Drug Administration. FDA Drug Safety Communication: reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drug, March 2010. Available at https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-reduced-effectiveness-plavix-clopidogrel-patients-who-are-poor. Accessed 7–20–2022.
Empey PE, Pratt VM, Hoffman JM, Caudle KE, Klein TE. Expanding evidence leads to new pharmacogenomics payer coverage. Genet Med. 2021;23(5):830–2.
Pharmacogenomic Testing Local Coverage Determination. Novitas Solutions. December 12, 2021. Available at: https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdId=39063&ver=11. Accessed 7/20/2022.
Weitzel KW, Elsey AR, Langaee TY, Burkley B, Nessl DR, Obeng AO, et al. Clinical pharmacogenetics implementation: approaches, successes, and challenges. Am J Med Genet C Semin Med Genet. 2014;166c(1):56–67.
Empey PE, Stevenson JM, Tuteja S, Weitzel KW, Angiolillo DJ, Beitelshees AL, et al. Multisite investigation of strategies for the implementation of CYP2C19 genotype-guided antiplatelet therapy. Clin Pharmacol Ther. 2018;104(4):664–74.
Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002–12.
Wittkowsky AK, Devine EB. Frequency and causes of overanticoagulation and underanticoagulation in patients treated with warfarin. Pharmacotherapy. 2004;24(10):1311–6.
Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S – e496.
Daly AK, King BP. Pharmacogenetics of oral anticoagulants. Pharmacogenetics. 2003;13(5):247–52.
Li T, Chang CY, Jin DY, Lin PJ, Khvorova A, Stafford DW. Identification of the gene for vitamin K epoxide reductase. Nature. 2004;427(6974):541–4.
Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352(22):2285–93.
Limdi NA, Wadelius M, Cavallari L, Eriksson N, Crawford DC, Lee MT, et al. Warfarin pharmacogenetics: a single VKORC1 polymorphism is predictive of dose across 3 racial groups. Blood. 2010;115(18):3827–34.
McDonald MG, Rieder MJ, Nakano M, Hsia CK, Rettie AE. CYP4F2 is a vitamin K1 oxidase: An explanation for altered warfarin dose in carriers of the V433M variant. Mol Pharmacol. 2009;75(6):1337–46.
Danese E, Montagnana M, Johnson JA, Rettie AE, Zambon CF, Lubitz SA, et al. Impact of the CYP4F2 p.V433M polymorphism on coumarin dose requirement: systematic review and meta-analysis. Clin Pharmacol Ther. 2012;92(6):746–56.
Perera MA, Cavallari LH, Limdi NA, Gamazon ER, Konkashbaev A, Daneshjou R, et al. Genetic variants associated with warfarin dose in African-American individuals: a genome-wide association study. Lancet (London, England). 2013;382(9894):790–6.
Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH, Nicholson T, et al. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med. 2013;369(24):2294–303.
Kimmel SE, French B, Kasner SE, Johnson JA, Anderson JL, Gage BF, et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med. 2013;369(24):2283–93.
Ramirez AH, Shi Y, Schildcrout JS, Delaney JT, Xu H, Oetjens MT, et al. Predicting warfarin dosage in European-Americans and African-Americans using DNA samples linked to an electronic health record. Pharmacogenomics. 2012;13(4):407–18.
Limdi NA, Brown TM, Yan Q, Thigpen JL, Shendre A, Liu N, et al. Race influences warfarin dose changes associated with genetic factors. Blood. 2015;126(4):539–45.
Gage BF, Bass AR, Lin H, Woller SC, Stevens SM, Al-Hammadi N, et al. Effect of genotype-guided warfarin dosing on clinical events and anticoagulation control among patients undergoing hip or knee arthroplasty: the GIFT randomized clinical trial. JAMA. 2017;318(12):1115–24.
Gage BF, Eby C, Johnson JA, Deych E, Rieder MJ, Ridker PM, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther. 2008;84(3):326–31.
Klein TE, Altman RB, Eriksson N, Gage BF, Kimmel SE, Lee MT, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360(8):753–64.
Coumadin (warfarin) Package Insert 2017. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/009218s118lbl.pdf Accessed 7/20/2022.
Jorgensen AL, Prince C, Fitzgerald G, Hanson A, Downing J, Reynolds J, et al. Implementation of genotype-guided dosing of warfarin with point-of-care genetic testing in three UK clinics: a matched cohort study. BMC Med. 2019;17(1):76.
Nutescu EA, Drozda K, Bress AP, Galanter WL, Stevenson J, Stamos TD, et al. Feasibility of implementing a comprehensive warfarin pharmacogenetics service. Pharmacotherapy. 2013;33(11):1156–64.
Roden DM, Van Driest SL, Mosley JD, Wells QS, Robinson JR, Denny JC, et al. Benefit of preemptive pharmacogenetic information on clinical outcome. Clin Pharmacol Ther. 2018;103(5):787–94.
Asiimwe IG, Pirmohamed M. Ethnic diversity and warfarin pharmacogenomics. Front Pharmacol. 2022;13:866058.
Davis BH, Limdi NA. Translational pharmacogenomics: discovery, evidence synthesis and delivery of race-conscious medicine. Clin Pharmacol Ther. 2021;110(4):909–25.
Muyambo S, Ndadza A, Soko ND, Kruger B, Kadzirange G, Chimusa E, et al. Warfarin pharmacogenomics for precision medicine in real-life clinical practice in Southern Africa: harnessing 73 variants in 29 pharmacogenes. OMICS. 2022;26(1):35–50.
Steiner HE, Giles JB, Patterson HK, Feng J, El Rouby N, Claudio K, et al. Machine learning for prediction of stable warfarin dose in US Latinos and Latin Americans. Front Pharmacol. 2021;12:749786.
Sosa-Macias M, Moya GE, A LL, Ramírez R, Terán E, Peñas LEM, et al. Population pharmacogenetics of Ibero-Latinoamerican populations (MESTIFAR 2014). Pharmacogenomics. 2015;16(7):673–6.
Nizamuddin S, Dubey S, Singh S, Sharma S, Machha P, Thangaraj K. CYP2C9 variations and their pharmacogenetic implications among diverse South Asian populations. Pharmacogenomics Pers Med. 2021;14:135–47.
Li D, Peng L, Xing S, He C, Jin T. Genetic analysis of pharmacogenomic VIP variants in the Wa population from Yunnan Province of China. BMC Genom Data. 2021;22(1):51.
O’Sullivan JW, Raghavan S, Marquez-Luna C, Luzum JA, Damrauer SM, Ashley EA, et al. Polygenic risk scores for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2022:101161cir0000000000001077.
National Human Genome Research Institute. Advancing Genomic Medicine Reserch. Request for proposal available at: https://grants.nih.gov/grants/guide/rfa-files/RFA-HG-20-036.html. Accessed 7/21/2022.
Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363(4):301–4.
Funding
Dr. Tuteja is supported by grants from the National Heart Lung Blood Institute (HL143161) and the Penn Center for Precision Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sony Tuteja declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tuteja, S. Application of Pharmacogenetics for the Use of Antiplatelet and Anticoagulant Drugs. Curr Cardiovasc Risk Rep 17, 27–38 (2023). https://doi.org/10.1007/s12170-022-00713-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12170-022-00713-y