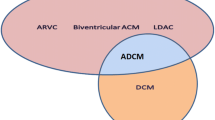
Abstract
Purpose of Review
Arrhythmogenic cardiomyopathy (ACM) represents a collection of cardiomyopathies with etiologies convergent upon highly arrhythmogenic clinical presentations not explained by ischemic, hypertensive, or valvular heart disease. This review describes the current state of knowledge regarding molecular mechanisms and genetic diagnosis in ACM.
Recent Findings
Clinical manifestations, specifically structural presentations, of ACM may span the right and left side of the heart. The umbrella definition of ACM encompasses disease mechanisms involving disruption of cell-cell adhesion, mechanotransduction, transcription, calcium handling, and post-transcriptional regulators. As such, the underlying genetics are themselves wide ranging but generally map to perturbations of proteins comprising the desmosome and regulating downstream cellular signaling. The recognition that many genetic forms of ACM share the feared outcomes of life-threatening ventricular arrhythmia and heart failure motivates the identification of causative genetic variants for family screening and in some cases precision therapy. In turn, contemporary clinical practice has shifted towards broadened genetic testing strategy and the discovery of complex gene-specific clinical ramifications.
Summary
The molecular mechanisms of ACM are complex, and the genetic architecture is ever evolving. Given this, multidisciplinary patient care for the incorporation of genetics and family cardiovascular care is critical. Future work will refine the understanding of gene-specific outcomes in ACM and implications for tailored therapy.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;318:129–33.
Marcus FI, Fontaine GH. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–98.
McKoy G, Protonotarios N. Identification of a deletion in Plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet. 2000;355:2119–24.
Carvajal-Huerta L. Epidermolytic palmoplantar keratoderma with woolly hair and dilated cardiomyopathy. J Am Acad Dermatol. 1998;39:418–21.
Corrado D, Basso C. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol. 1997;30:1512–20.
Sen-Chowdhry S, Syrris P. Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity. J Am Coll Cardiol. 2008;52:2175–87.
Towbin JA, et al. HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019, 2019. https://doi.org/10.1016/j.hrthm.2019.05.007Seminal guidelines providing contemporary review of etiology and management of arrhythmogenic cardiomyopathy.
Chatterjee D, et al. An autoantibody identifies arrhythmogenic right ventricular cardiomyopathy and participates in its pathogenesis. Eur Heart J. 2018;39:3932–44 Findings from this paper suggest a role for autoimmunity in the disease pathogenesis of arrhythmogenic cardiomyopathy.
Smith ED, et al. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation. 2020;141:1872–84 The findings of this paper highlight the gene-level specificity of certain ACM-causing genes, in addition to the role of inflammation in ACM.
Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Eur Heart J. 2010;31:806–14.
Kumar S, Baldinger SH. Long-term arrhythmic and nonarrhythmic outcomes of lamin A/C mutation carriers. J Am Coll Cardiol. 2016;68:2299–307.
James CA, Bhonsale A. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy—associated desmosomal mutation carriers. J Am Coll Cardiol. 2013;62:1290–7.
Ingles J, et al. Concealed arrhythmogenic right ventricular cardiomyopathy in sudden unexplained cardiac death events. Circ Genom Precis Med. 2018;11:e002355.
Borrmann CM, et al. The area composita of adhering junctions connecting heart muscle cells of vertebrates. II. Colocalizations of desmosomal and fascia adhaerens molecules in the intercalated disk. Eur J Cell Biol. 2006;85:469–85.
Zhao G, Qiu Y, Zhang HM, Yang D. Intercalated discs: cellular adhesion and signaling in heart health and diseases. Heart Fail Rev. 2019;24:115–32.
Garcia-Gras E, Lombardi R. Suppression of canonical Wnt/β-catenin signaling by nuclear Plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest. 2006;116:2012–21.
Kant S, Krusche CA, Gaertner A, Milting H, Leube RE. Loss of Plakoglobin immunoreactivity in intercalated discs in arrhythmogenic right ventricular cardiomyopathy: protein mislocalization versus epitope masking. Cardiovasc Res. 2016;109:260–71.
Hoorntje ET, te Rijdt WP. Arrhythmogenic cardiomyopathy: pathology, genetics, and concepts in pathogenesis. Cardiovasc Res. 2017;113:1521–31.
Chen SN, Gurha P. The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ Res. 2014;114:454–68.
Cerrone M, Remme CA, Tadros R, Bezzina CR, Delmar M. Beyond the one gene-one disease paradigm: complex genetics and pleiotropy in inheritable cardiac disorders. Circulation. 2019;140:595–610 This review describes the evolving understanding of the genetic architecture of inherited cardiovascular disease.
Wilde AAM, Amin AS. Clinical Spectrum of SCN5A Mutations: long QT syndrome, Brugada Syndrome, and Cardiomyopathy. JACC Clin Electrophysiol. 2018;4:569–79.
Ben-Haim Y, Asimaki A, Behr ER. Brugada syndrome and arrhythmogenic cardiomyopathy: overlapping disorders of the connexome? Europace. 2020. https://doi.org/10.1093/europace/euaa277.
Deo M, et al. KCNJ2 mutation in short QT syndrome 3 results in atrial fibrillation and ventricular proarrhythmia. Proc Natl Acad Sci U S A. 2013;110:4291–6.
Fidler LM, et al. Abnormal connexin43 in arrhythmogenic right ventricular cardiomyopathy caused by Plakophilin-2 mutations. J Cell Mol Med. 2009;13:4219–28.
Beyer EC, Paul DL, Goodenough DA. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987;105:2621–9.
Lyon RC, et al. Connexin defects underlie arrhythmogenic right ventricular cardiomyopathy in a novel mouse model. Hum Mol Genet. 2014;23:1134–50.
Bermúdez-Jiménez FJ, et al. Novel Desmin mutation p.Glu401Asp impairs filament formation, disrupts cell membrane integrity, and causes severe arrhythmogenic left ventricular cardiomyopathy/dysplasia. Circulation. 2018;137:1595–610.
Cowan J, Li D, Gonzalez-Quintana J, Morales A, Hershberger RE. Morphological analysis of 13 LMNA variants identified in a cohort of 324 unrelated patients with idiopathic or familial dilated cardiomyopathy. Circ Cardiovasc Genet. 2010;3:6–14.
Begay RL, Graw SL, Sinagra G, Asimaki A, Rowland TJ, Slavov DB, et al. Filamin C truncation mutations are associated with arrhythmogenic dilated cardiomyopathy and changes in the cell-cell adhesion structures. JACC Clin Electrophysiol. 2018;4:504–14.
Ortiz-Genga MF, et al. Truncating FLNC mutations are associated with high-risk dilated and arrhythmogenic cardiomyopathies. J Am Coll Cardiol. 2016;68:2440–51.
Goodyer WR, et al. Broad genetic testing in a clinical setting uncovers a high prevalence of Titin loss-of-function variants in very early onset atrial fibrillation. Circulation: Genomic and Precision Medicine. 2019;12:e002713.
Parikh VN, et al. Regional variation in RBM20 causes a highly penetrant arrhythmogenic cardiomyopathy. Circ Heart Fail. 2019;12:e005371.
Mestroni L, Sbaizero O. Arrhythmogenic cardiomyopathy: mechanotransduction going wrong. Circulation. 2018;137:1611–3.
Ruwhof C, van der Laarse A. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res. 2000;47:23–37.
Craige SM, Chen K, Blanton RM, Keaney JF Jr, Kant S. JNK and cardiometabolic dysfunction. Biosci Rep. 2019;39:BSR20190267.
Basso C, Thiene G, Corrado D, Angelini A, Nava A, Valente M. Arrhythmogenic right ventricular cardiomyopathy. Circulation. 1996;94:983–91.
Kerkar A, et al. Pathological overlap of arrhythmogenic right ventricular cardiomyopathy and cardiac sarcoidosis. Circulation: Genomic and Precision Medicine. 2019;12:e002638.
Delmar M, McKenna WJ. The cardiac desmosome and arrhythmogenic cardiomyopathies: from gene to disease. Circ Res. 2010;107:700–14.
Gao S, Puthenvedu D, Lombardi R, Chen SN. Established and emerging mechanisms in the pathogenesis of arrhythmogenic cardiomyopathy: a multifaceted disease. Int J Mol Sci. 2020;21:6320 This review details key molecular pathways in the disease pathogenesis of ACM.
Protonotarios N, Tsatsopoulou A, Patsourakos P, Alexopoulos D, Gezerlis P, Simitsis S, et al. Cardiac abnormalities in familial palmoplantar keratosis. Br Heart J. 1986;56:321–6.
Norgett EE, et al. Recessive mutation in Desmoplakin disrupts Desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Genet. 2000;9:2761–6.
Richards S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–23.
Caleshu, C. et al. Lack of specificity of ACMG classification rules decreases inter-curator concordance. ClinGen’s adaptation of ACMG’s framework to standardize interpretation of MYH7 related cardiomyopathy variants. 2015. in Poster presented at the 65th meeting of the American Society of Human Genetics, Baltimore, MD, USA 6–10 (2015).
Kelly MA, et al. Adaptation and validation of the ACMG/AMP variant classification framework for MYH7-associated inherited cardiomyopathies: recommendations by ClinGen’s inherited cardiomyopathy expert panel. Genet Med. 2018;20:351–9.
Walsh R, et al. Reassessment of Mendelian gene pathogenicity using 7,855 cardiomyopathy cases and 60,706 reference samples. Genet Med. 2017;19:192–203 This paper employs a unique, population-based case-control approach to understanding the genetic architecture of inherited cardiomyopathy genes.
Domínguez F, et al. Dilated Cardiomyopathy due to BLC2-associated athanogene 3 (BAG3) Mutations. J Am Coll Cardiol. 2018;72:2471–81.
Chami N, et al. Nonsense mutations in BAG3 are associated with early-onset dilated cardiomyopathy in French Canadians. Can J Cardiol. 2014;30:1655–61.
Den Haan AD, et al. Comprehensive desmosome mutation analysis in North Americans with arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Cardiovasc Genet. 2009;2:428–35.
Bhuiyan ZA, Jongbloed JDH, van der Smagt J, Lombardi PM, Wiesfeld ACP, Nelen M, et al. Desmoglein-2 and desmocollin-2 mutations in dutch arrhythmogenic right ventricular dysplasia/cardiomypathy patients: results from a multicenter study. Circ Cardiovasc Genet. 2009;2:418–27.
Hof IE, van der Heijden JF, Kranias EG, Sanoudou D, de Boer RA, van Tintelen JP, et al. Prevalence and cardiac phenotype of patients with a phospholamban mutation. Neth Hear J. 2019;27:64–9.
Te Rijdt WP, et al. Phospholamban p.Arg14del cardiomyopathy is characterized by phospholamban aggregates, aggresomes, and autophagic degradation. Histopathology. 2016;69:542–50.
Li W, et al. SCN5A Variants: association with cardiac disorders. Front Physiol. 2018;9:1372.
Crasto S, My I, Di Pasquale E. The broad spectrum of LMNA cardiac diseases: from molecular mechanisms to clinical phenotype. Front Physiol. 2020;11:761.
Chen SN, Sbaizero O, Taylor MRG, Mestroni L. Lamin A/C cardiomyopathy: implications for treatment. Curr Cardiol Rep. 2019;21:160.
Mazzarotto F, Tayal U, Buchan RJ, Midwinter W, Wilk A, Whiffin N, et al. Reevaluating the genetic contribution of monogenic dilated cardiomyopathy. Circulation. 2020;141:387–98.
Hedberg C, Melberg A, Kuhl A, Jenne D, Oldfors A. Autosomal dominant myofibrillar myopathy with arrhythmogenic right ventricular cardiomyopathy 7 is caused by a DES mutation. Eur J Hum Genet. 2012;20:984–5.
Béhin A, et al. Myofibrillar myopathies: state of the art, present and future challenges. Rev Neurol. 2015;171:715–29.
Lopez-Ayala JM, Ortiz-Genga M, Gomez-Milanes I, Lopez-Cuenca D, Ruiz-Espejo F, Sanchez-Munoz JJ, et al. A mutation in the Z-line Cypher/ZASP protein is associated with arrhythmogenic right ventricular cardiomyopathy. Clin Genet. 2015;88:172–6.
Merner ND, Hodgkinson KA, Haywood AFM, Connors S, French VM, Drenckhahn JD, et al. Arrhythmogenic right ventricular cardiomyopathy type 5 is a fully penetrant, lethal arrhythmic disorder caused by a missense mutation in the TMEM43 gene. Am J Hum Genet. 2008;82:809–21.
Sveinbjornsson G, et al. Variants in NKX2-5 and FLNC cause dilated cardiomyopathy and sudden cardiac death. Circ Genom Precis Med. 2018;11:e002151.
Schott JJ, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP, et al. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science. 1998;281:108–11.
Krahn AD, et al. The Canadian Arrhythmogenic Right Ventricular Cardiomyopathy Registry: rationale, design, and preliminary recruitment. Can J Cardiol. 2016;32:1396–401.
Hershberger RE, Givertz MM, Ho CY, Judge DP, Kantor PF, McBride KL, et al. Genetic evaluation of cardiomyopathy—a heart failure society of America Practice Guideline. J Card Fail. 2018;24:281–302.
Brittney M, et al. Influence of panel selection on yield of clinically useful variants in arrhythmogenic right ventricular cardiomyopathy families. Circulation: Genomic and Precision Medicine. 2020;13:548–50.
Isbister JC, et al. ‘Concealed cardiomyopathy’ as a cause of previously unexplained sudden cardiac arrest. Int J Cardiol. 2020. https://doi.org/10.1016/j.ijcard.2020.09.031.
Strande NT, Riggs ER, Buchanan AH, Ceyhan-Birsoy O, DiStefano M, Dwight SS, et al. Evaluating the clinical validity of gene-disease associations: an evidence-based framework developed by the clinical genome resource. Am J Hum Genet. 2017;100:895–906.
MacArthur DG, Manolio TA, Dimmock DP, Rehm HL, Shendure J, Abecasis GR, et al. Guidelines for investigating causality of sequence variants in human disease. Nature. 2014;508:469–76.
Biesecker LG, Nussbaum RL, Rehm HL. Distinguishing variant pathogenicity from genetic diagnosis. JAMA. 2018;320:1929.
Pugh TJ, Kelly MA, Gowrisankar S, Hynes E, Seidman MA, Baxter SM, et al. The landscape of genetic variation in dilated cardiomyopathy as surveyed by clinical DNA sequencing. Genet Med. 2014;16:601–8.
Headrick AT, Rosenfeld JA, Yang Y, Tunuguntla H, Allen HD, Penny DJ, et al. Incidentally identified genetic variants in arrhythmogenic right ventricular cardiomyopathy-associated genes among children undergoing exome sequencing reflect healthy population variation. Mol Genet Genomic Med. 2019;7:e593.
Reuter CM, et al. A new approach to rare diseases of children: the undiagnosed diseases network. J Pediatr. 2018;196:291–297.e2.
Splinter K, et al. Effect of genetic diagnosis on patients with previously undiagnosed disease. N Engl J Med. 2018;379:2131–9.
Mellor G, et al. Genetic testing in the evaluation of unexplained cardiac arrest: from the CASPER (Cardiac Arrest Survivors With Preserved Ejection Fraction Registry). Circ Cardiovasc Genet. 2017;10:e001686.
Bagnall RD, Ingles J, Dinger ME, Cowley MJ, Ross SB, Minoche AE, et al. Whole genome sequencing improves outcomes of genetic testing in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2018;72:419–29.
Cirino AL, et al. A comparison of whole genome sequencing to multigene panel testing in hypertrophic cardiomyopathy patients clinical perspective. Circulation: Genomic and Precision Medicine. 2017;10:e001768.
Groeneweg JA, Bhonsale A, James CA, te Riele AS, Dooijes D, Tichnell C, et al. Clinical presentation, long-term follow-up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members. Circ Cardiovasc Genet. 2015;8:437–46.
Hasselberg NE, Haland TF, Saberniak J, Brekke PH, Berge KE, Leren TP, et al. Lamin A/C cardiomyopathy: young onset, high penetrance, and frequent need for heart transplantation. Eur Heart J. 2018;39:853–60.
van Berlo JH, et al. Meta-analysis of clinical characteristics of 299 carriers of LMNA gene mutations: do lamin A/C mutations portend a high risk of sudden death? J Mol Med. 2005;83:79–83.
Pasotti M, et al. Long-term outcome and risk stratification in dilated cardiolaminopathies. J Am Coll Cardiol. 2008;52:1250–60.
Brun F, Gigli M, Graw SL, Judge DP, Merlo M, Murray B, et al. FLNC truncations cause arrhythmogenic right ventricular cardiomyopathy. J Med Genet. 2020;57:254–7.
Truszkowska GT, Bilińska ZT, Kosińska J, Śleszycka J, Rydzanicz M, Sobieszczańska-Małek M, et al. A study in Polish patients with cardiomyopathy emphasizes pathogenicity of phospholamban (PLN) mutations at amino acid position 9 and low penetrance of heterozygous null PLN mutations. BMC Med Genet. 2015;16:21.
Cox MGPJ, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: pathogenic desmosome mutations in index-patients predict outcome of family screening: Dutch arrhythmogenic right ventricular dysplasia/cardiomyopathy genotype-phenotype follow-up study. Circulation. 2011;123:2690–700.
Carruth ED, et al. Prevalence and electronic health record-based phenotype of loss-of-function genetic variants in arrhythmogenic right ventricular cardiomyopathy-associated genes. Circ Genom Precis Med. 2019;12:e002579.
Hall CL, et al. Frequency of genetic variants associated with arrhythmogenic right ventricular cardiomyopathy in the genome aggregation database. Eur J Hum Genet. 2018;26:1312–8.
Poller W, et al. Familial recurrent myocarditis triggered by exercise in patients with a truncating variant of the Desmoplakin gene. J Am Heart Assoc. 2020;9:e015289.
Arscott P, Caleshu C, Kotzer K, Kreykes S, Kruisselbrink T, Orland K, et al. A case for inclusion of genetic counselors in cardiac care. Cardiol Rev. 2016;24:49–55.
Ison HE, Ware SM, Schwantes-An TH, Freeze S, Elmore L, Spoonamore KG. The impact of cardiovascular genetic counseling on patient empowerment. J Genet Couns. 2019;28:570–7.
Funding
This work was supported by the Boring Trust Research Award (to AMD), the John Taylor Babbitt Foundation, the Sarnoff Cardiovascular Research Foundation, and NIH National Heart Lung and Blood Institute K08 HL143185 (to VNP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Annika M. Dries and Victoria N. Parikh declare that they have no conflict of interest. Chloe M. Reuter is a consultant for My Gene Counsel.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any
of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Genetics
Rights and permissions
About this article
Cite this article
Reuter, C.M., Dries, A.M. & Parikh, V.N. Arrhythmogenic Cardiomyopathy: Mechanisms, Genetics, and Their Clinical Implications. Curr Cardiovasc Risk Rep 15, 7 (2021). https://doi.org/10.1007/s12170-021-00669-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s12170-021-00669-5