Abstract
Roasting is a crucial and essential step to produce quality coffee. However, it could lead to the formation of toxic and suspected carcinogenic or procancerogenic compounds, such as polycyclic aromatic hydrocarbons. In this work, a simple and easily automatable green procedure based on solid-phase microextraction coupled with gas chromatography for the analysis of acenaphthene, anthracene, benzo[ghi]perylene, benzo[a]pyrene, chrysene, fluoranthene, fluorene, naphthalene, and pyrene, in dark roasted and decaffeinated commercial coffees, was developed. The method was optimized for the determination of the analytes both in solid samples, such as ground coffee or coffee grounds, and liquids, such as espresso coffee, using a polyacrylate-coated fused silica fiber (85 μm) by direct immersion. The performance of the analytical method, developed in terms of sensitivity, reproducibility, and recoveries, proved to be suitable for the applications. Among the 9 polycyclic aromatic hydrocarbons investigated in the selected coffees, chrysene and pyrene were the most representative congeners with values ranging from undetectable to 95.6 ± 11 ng/g for chrysene and from undetectable to 404.7 ± 42.0 ng/g for pyrene. Benzo[a]pyrene was detected in two samples of dark roasted coffee which therefore had the highest toxicity/carcinogenicity in terms of toxic equivalent. The estimated limit of detection for benzo[a]pyrene in ground coffee and coffee grounds was 9.0 ng/g. About 30% of the PAHs were transferred to the infusion while the remaining part was retained by the coffee grounds.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Roasting is a crucial step for the manufacture of coffee, as it affects the development of color, aroma, and flavor, which are essential for the product quality (Jimenez et al. 2014; Binello et al. 2021). Therefore, some conditions of the roasting step, such as temperature and time, must be carefully optimized and controlled. In general, temperatures between 220 and 250 °C are applied in an electric oven for indirect roasting of green beans, but higher temperatures could be easily reached when the flame, charcoal grill, or gas oven is applied directly to the coffee beans (Houessou et al. 2007; Orecchio et al. 2009). As a result of the intense thermal processes, partial carbonizations could occur with the formation of undesirable compounds, such as polycyclic aromatic hydrocarbons (PAHs) (Rey-Salgueiro et al. 2008; dos Santos et al. 2019), a vast class of toxic and carcinogenic or procarcinogenic ubiquitous compounds (Jimenez et al. 2014; Gao et al. 2018) that can cause cumulative and unpredictable harm to human health, as one can be randomly and constantly exposed to various mixtures of them throughout the life (Jalili et al. 2020).
Naphthalene, whose structure is characterized by two aromatic rings, is considered the simplest PAH, while phenanthrene and anthracene are tricyclic species. These compounds are classified as light PAHs (Purcaro et al. 2013). However, the number of condensed aromatic rings can increase from four to six, resulting in the formation of many isomeric compounds known as heavy PAHs. In general, the aqueous solubility of PAHs decreases with increasing condensed rings (Johnsen et al. 2005). Hence, light PAHs are more readily available for biological absorption and degradation than heavy PAHs (Haritash and Kaushik 2009; Choi et al. 2010). As for the volatility, PAHs with two or four rings are semi-volatile compounds, while those with five or more rings are characterized by low volatility and are more persistent in the environment (Haritash and Kaushik 2009; Choi et al. 2010).
It was suggested that the transformation of light PAHs into heavy PAHs in coffee samples occurs as the degree of roasting increases (Orecchio et al. 2009). In fact, phenanthrene, anthracene, benzo[a]anthracene, and benzo[g,h,i]perylene were found in coffee beans roasted at temperatures above 220 °C, while the formation of pyrene and chrysene was observed after a thermal treatment at 260 °C (Orecchio et al. 2009). Additionally, coffee can also be contaminated by PAHs through the deposition of airborne particles on plants, growth in contaminated soils or contaminated water, or through packaging materials (Houessou et al. 2007; Orecchio et al. 2009). Moreover, PAHs can also be transferred from solid samples to infusion (< 35%), with slightly lower extractability for dark roasted coffee than for light roasted coffee (Houessou et al. 2007) .
To minimize human exposure, PAHs have been evaluated as contaminants by numerous international organizations (Binello et al. 2021). Some PAHs like benzo[a]pyrene, benzo[a]anthracene, and chrysene have been expressed as markers in food by the European Commission (Zelinkova and Wenzl 2015), and maximum permitted concentrations have been established from 1 to 30 µg/kg in different food products. The maximum levels of PAHs have not yet been determined by government organizations for coffee and tea-based beverages (Zelinkova and Wenzl 2015). Therefore, analytical methods capable of determining PAHs at trace levels in such matrices are highly required.
Two techniques are currently most frequently employed to determine PAHs, namely liquid chromatography (LC) coupled with different detectors (mostly fluorescence and UV) and gas chromatography coupled to mass spectrometry (GC/MS) (Jalili et al. 2020; Binello et al. 2021). Given the complexity of the food matrices and the low levels of PAH contamination, extraction, purification, and preconcentration steps are always needed before instrumental analysis. Numerous approaches have been proposed over the years, from conventional solid liquid extraction (SLE) or liquid–liquid extraction (LLE) to solid-phase extraction (SPE) or solid-phase microextraction (SPME) (Jalili et al. 2020; Binello et al. 2021). However, conventional and SPE approaches are definitely not “green” and poorly effective due to the hydrophobic characteristics of PAHs. On the contrary, being simple, solventless, rapid, and reusable, SPME represents a very good alternative. It uses a fiber of reduced volume and thickness coated with a suitable extractive polymer phase and has found wide application in the food and environmental fields for the extraction of polar and non-polar compounds from water samples (Vas and Vekey 2004; Zambonin and Aresta 2021). Since, the role of the polymeric phase is crucial in the selective extraction of target compounds (Pawliszyn 2012), SPME fibers with different coating materials are commercially available. Polydimethylsiloxane (PDMS), carboxen/PDMS (CAR/PDMS), and divinylbenzene/CAR/PDMS (DVB/CAR/PDMS) are preferred coatings for the determination of PAHs because they offer adequate sensitivity and good recoveries (Jalili et al. 2020). However, new SPME coating materials with high selectivity, greater stability, and high extraction capacity have been recently developed for the determination of PAHs in food. Yazdi et al. (2018) proposed a hollow fiber extraction followed by LC/UV analysis for PAH determination in coffee and tea beverages, dos Santos et al. (2019) used a cold fiber for the SPME-GC/MS determination of PAHs and their nitrated and oxygenated derivatives in coffee brews, while Erdem et al. (2021) introduced a montmorillonite/chitosan/dicationic ionic liquid fiber for the GC–MS determination of 16 PAHs in coffee and tea drinks. Although the new SPME coatings are very advantageous for the extraction of PAHs from coffee and tea samples, their manufacture in the laboratory, unlike traditional commercial fibers, could be laborious and unreproducible and could lead to unreliable results.
Very recently, the ability of the commercial polyacrylate (PA) SPME fiber to effectively extract selected PAHs was reported (Aresta et al. 2022) in a work focused on their determination in soot samples by ultra-high-performance LC with UV-photodiode array detection (UHPLC-PDA). Nevertheless, the PA fiber has never been applied to the extraction of PAHs in coffee samples.
In the present paper, direct immersion (DI) SPME using a 85 µm PA fiber was optimized for the extraction of nine selected PAHs recognized as genotoxic and carcinogenic (acenaphthene (ACE), anthracene (ANT), benzo[ghi]perylene (BPR), benzo[a]pyrene (BaP), chrysene (CHR), fluoranthene (FLR), fluorene (FLN), naphthalene (NPH), and pyrene (PRN)). The best extraction conditions were then adopted for the determination of the target analytes in commercial solid and liquid coffee samples.
Materials and Methods
Materials
Acenaphthene (ACE), anthracene (ANT), benzo[ghi]perylene (BPR), benzo[a]pyrene (BaP), chrysene (CHR), fluoranthene (FLR), fluorene (FLN), naphthalene (NPH), and pyrene (PRN) were purchased from Sigma-Aldrich (MI, Italy). Stock solutions (0.25 mg/mL) were prepared in ethanol and stored at 8 °C.
Solid-Phase Microextraction
Sigma-Aldrich supplied the SPME polyacrylate fibers (85 µm), which was conditioned before use according to the instructions. Analyte working solutions were prepared in 15 mL amber glass vials with screw cap and pierceable septum (PTFA) (Supelco, MI, Italy) using HPLC-grade water (Sigma-Aldrich). Extractions were carried out at room temperature for 30 min under magnetic stirring (800 rpm) using a cylindrical stirred bar (10 × 4 mm, Supelco). Desorption was carried out in the GC injector for 1 min at 250 °C. All experiments were conducted in triplicate.
Apparatus and Instrumental Conditions
The GC/MS system was a Finnigan TRACE GC ultra-gas chromatograph (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a split/splitless injector, interfaced to an ion-trap MS (Finnigan Polaris Q, Thermo Fisher Scientific). The capillary column was a Supelco SPB-5 fused silica (30 m, 0.25 μm i.d., 0.25 μm film thickness) with helium (purity 99.999%, Sol spa, Monza, Italy) as carrier gas (flow rate 1 mL/min). The temperature of the transfer line was 300 °C, while the injector (splitless mode) was kept at 250 °C. The oven temperature program was 60 °C held for 1 min; 10 °C/min from 60 to 300 °C; 300 °C held for 15 min. The mass spectrometer was operated in the electron impact positive ion mode (EI +) with the ion source temperature set at 250 °C. The electron energy was 70 eV, and the filament current 150 μA. As specified in the text, the acquisition mode used was as follows: total ion current (TIC, m/z range 50–350) or selected ion monitoring (SIM; the m/z ions useful for the quantification were: 128 (NPH, tR 9.19 min), 154 (ACE, 13.42 min), 166 (FLN, 14.67 min), and 178 (ANT, 17.08 min) up to 20 min, then 202 (FLR, 20.38; PRN, 20.53 min), 228 (CHR, 23.60 min), 252 (BaP, 26.30 min), and 276 (BPR, 31.90 min) until the end of the chromatographic run). The identification of the analytes was based on both the correspondence of the retention times with those of reference standard and on NIST library matches (match factors always > 900).
Sample Preparation
Ground coffee samples (3 different brands of dark roasted and 2 of decaffeinated) were purchased from a local market (Bari, Italy). Each solid sample (72 mg) was directly weighed into a 15 mL amber vial and then 1.5 mL of ethanol was added. After the insertion of a magnetic stir bar, the vial was sealed and placed on a magnetic plate, and the solution stirred for 30 min. Then, 13.5 mL of HPLC water was added through the septum with a syringe, and the sample was finally subjected to DI-SPME. The coffee infusions (19 mL per 7.5 g solid) were prepared with an espresso machine, and the related coffee grounds were recovered after each preparation. An aliquot of each infusion (8.5 mL) was promptly transferred into an amber vial containing 1.5 mL of ethanol and a magnetic bar. After closing the vial and shaking the solution for 30 min, 5.0 mL of water was added through the septum and the sample finally subjected to SPME. Coffee grounds were instead weighed (140 mg, ≈ 72 mg of ground coffee) and subjected to the same procedure described for ground coffee samples.
For method validation, PAH-free coffee samples were obtained performing a series of repeated extractions. Briefly, 5 g of ground coffee was extracted 5 folds with 50 mL of an acetonitrile/methanol/2-propanol (15:65:20, v/v/v) mixture. After centrifugation (3500 rpm, 5 min), the resulting PAHs free coffee samples were dried and divided into aliquots. Some were used as blank; others were added with variable amounts of PAHs to cover the concentration range 0.5–1,000 µg/g for the calculation of calibration curves, coefficient of variations, and sensibility. The limits of detection (LODs) were experimentally obtained by decreasing the concentration of the analytes until a signal to noise ratio (S/N) of 3 was obtained. The limits of quantification (LOQs) were estimated as 10/3 times the LODs. Both limits were experimentally verified by adding standard solutions at the expected concentrations to PAH-free samples that were then analyzed using the optimized procedure.
For recovery and repeatability studies, PAH-free coffee samples were spiked with PAH standard solutions at concentration levels of 50, 100, and 200 ng/g. Three replicates for each concentration level were performed. Recoveries were calculated as the ratios between the peak areas obtained for the target PAHs in spiked samples and in standard solutions, respectively, at the same concentration levels. The within-day and between-days repeatability (% RSD) was performed by repeated analysis of the same samples for 3 times in 1 day and for 4 consecutive days, respectively.
Estimation of the Toxicity/Carcinogenicity of PAHs
The estimations were calculated using the following equation:
where TEQ is the toxic equivalent of the sample; PAHi is the concentration of each PAH; TEFi is the toxic equivalent factor of each PAH from the list reported by Nisbet and LaGoy (1992) which is 0.001 for acenaphthene, fluoranthene, fluorene, naphthalene, and pyrene; 0.01 for anthracene, benzo[ghi]perylene, and chrysene; and 1.0 for benzo[a]pyrene.
Results
First, the extraction kinetics of acenaphthene (ACE), anthracene (ANT), benzo[ghi]perylene (BPR), benzo[a]pyrene (BaP), chrysene (CHR), fluoranthene (FLR), fluorene (FLN), naphthalene (NPH), and pyrene (PRN) were obtained reporting the area counts, obtained by the SPME-GC/MS analyses of a PAH working solution (1 µg/mL), versus the extraction time. The relevant results are shown in Fig. 1.
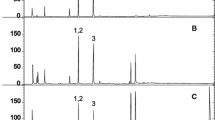
Then, the analytical performance of the DI-SPME-GC/MS (SIM) method was assessed in blank samples fortified with the target PAHs at appropriate concentration levels. Figure 2 shows a typical chromatogram of a spiked blank sample. Table 1 reports the validation parameters obtained by fortifying a blank sample of PAH-free coffee. Table 2 reports the information relevant to average recoveries (%), repeatability (% RSD), limits of detection (LOQs, ng/g), and quantification (LODs, ng/g), obtained with the DI-SPME-GC/MS method in spiked PAH-free coffee sample.
The results of the repeatability studies showed that there were no statistically significant changes (F test, p < 0.05) between within-day (n = 3) and between-days (n = 3, 4 days) data, while a slight variability was observed between the three concentration levels tested (50, 100, and 200 ng/g). Finally, several dark roasted and decaffeinated coffee samples were analyzed for the determination of the target compounds. The concentration levels of the selected PAHs found in all the analyzed samples are reported in Table 3. The carcinogenic potency of the considered PAHs in the coffee samples was also estimated in terms of TEQ. The calculated values are also listed in Table 3.
Discussion
Polycyclic aromatic hydrocarbons (PAHs) are of great concern due to their carcinogenicity and persistence in nature. Therefore, as reported in the “Introduction,” numerous analytical methods have been proposed for their determination in different matrices (environmental, food and biological). Direct immersion-solid–phase microextraction followed by gas chromatography/mass spectrometry has proven to be a great option for their determination (Vas and Vekey 2004; Pawliszyn 2012). For this reason, this procedure was preferred to the headspace (HS) SPME approach for the extraction of ACE, ANT, BPR, BaP, CHR, FLR, FLN, NPH, and PRN from water samples, in which 2e6-ring PAHs can be poorly soluble and strongly bound to matrix components (Naccarato et al. 2018). Since plastics, especially polyethylene, can be the cause of PAH loss during sample pretreatment due to analyte adsorption (Naccarato et al. 2018), they were completely avoided in this study. The samples were also protected from light by using amber vials as a further precaution. Finally, to increase the solubility of the analytes, the addition of low amounts of ethanol (1%) to the samples was considered.
The polyacrylate coating for SPME fibers has a good selectivity for aromatic compounds and is recommended by the supplier for the extraction of insecticides and polar semi-volatile compounds from water samples (Vas and Vekey 2004). Besides, it has already been shown to successfully extract these pollutants from complex matrices by the direct immersion approach (Aresta et al. 2022). Therefore, the PA coating (85 µm, thickness) was selected from those available on the market to extract the selected PAHs from coffee samples.
During the development of a SPME procedure, some parameters, such as the effect of pH and ionic strength, need to be optimized to improve the selectivity and efficiency of the extraction, which depend also on the properties of the target analytes. However, the optimization of pH and ionic strength was not performed in this case due to the physicochemical characteristics of the selected PAHs. In fact, the increase in ionic strength would seem to have a negative effect on the DI-SPME of these compounds (Pacheco-Fernandez et al. 2020; Naccarato et al. 2018).
Under the selected conditions (ambient temperature, no salt, no pH correction), the extraction profiles shown in Fig. 1 were obtained. When the fiber is exposed to the sample, the partition of the analytes between the matrix and the stationary phase begins until equilibrium is reached, when the amount of analyte extracted remains constant and corresponds to the amount extracted after an infinite time, within the limits of the experimental error. The minimum time required is named equilibrium time. As can be seen in Fig. 1, ACE, ANT, FLN, and NPH showed equilibrium times greater than 60 min, while the other PAHs, which are characterized by shorter equilibrium times, were characterized by a decrease in the area counts after a maximum. The size, shape, and solubility of a molecule are important factors in the extraction process that can affect its extraction kinetics (Vas and Vekey 2004). The selected PAHs have flat planar structures, 2e6 rings, no substituent groups, and can interact through π-π interactions both with the fiber coating phase and with the medium (Naccarato et al. 2018). Their molecular weight determines the rate at which they can move in and out of the coating and through the sample, while solubility affects the strength of the bond with the polymer and of attraction between molecules. Both factors produce competitive adsorption processes which are likely responsible for the abovementioned decreases in peak areas. Due to this experimental evidence and also to avoid prolonged extractions, 30 min is selected as the best extraction time, since it is possible to work in non-equilibrium conditions if the time is carefully controlled (Pawliszyn 2012). In general, 60 min is evaluated as the best extraction time for PDMS (100 µm), CAR/PDMS (65 µm), and DVB/CAR/PDMS (50/30 µm).
As observable in Tables 1 and 2, the method has a wide linear range, and sensitivity and reproducibility are adequate for the determination of PAHs in coffee samples. The LODs ranged from 1.4 (ACE) to 79.6 ng/g (BPR) and from 0.3 (ACE) to 16.6 ng/g (BPR), respectively, and are below the maximum permitted concentrations (1–30 ng/g) (Zelinkova and Wenzl 2015). Lower sensitivities for PAHs in coffee samples were obtained with new coating materials (Yazdi et al. 2018; dos Santoset al. 2019; Huang et al. 2019; Erdem et al. 2021), even if the performances of these new fibers may be lacking due to their lab-made preparation, not guaranteed by a supplier.
Recoveries were concentration-dependent and ranged from 67 to 99% at the level of 50 ng/g, from 79 to 99% at 100 ng/g, and from 59 to 87% at 200 ng/g. The slight decrease observed at higher concentrations may be related to the low water solubility of the analytes and their interactions with matrix components. In any case, the relative recoveries obtained with the PA fiber in this work are comparable to those reported by extracting PAHs from coffee samples using other commercial or lab-made coatings (Yazdi et al. 2018; dos Santoset al. 2019; Erdem et al. 2021). In the light of the evidence reported in the literature and the validation parameters obtained with the optimized procedure, the developed method provided the reliable determination of PAHs in coffee samples.
The analytical results in Table 3 show that chrysene and pyrene were the most abundant PAHs in the coffee samples examined, indicating that roasting of those samples probably occurred at very high temperatures, i.e., ≥ 280 °C (Orecchio et al. 2009). The decaffeinated roasted samples showed the higher concentration of pyrene. Among the samples tested, only two (dark roasted powder numbers 2 and 3) were found to be contaminated with BaP, at concentration levels comparable to its estimated LOD (9.0 ng/g). It is worth noting that according to literature data, about 30% of the PAHs were transferred to the infusion, while the remaining part was retained in the coffee ground.
The maximum toxicity/carcinogenicity of the considered coffee samples was associated to those samples characterized by the presence of benzo[a]pyrene. Given the TAF values attributed to the different PAHs (Nisbet and LaGoy 1992), those coffee samples in which chrysene and pyrene were more representative showed TEQ several times lower than those with BaP (Table 3).
Conclusions
This study reports for the first time the successful use of a polyacrylate-coated silica SPME fiber (85 µm) for the extraction and subsequent analysis of 9 PAHs, i.e., acenaphthene, anthracene, benzo[ghi]perylene, benzo[a]pyrene, chrysene, fluoranthene, fluorene, naphthalene, and pyrene, from coffee samples (ground coffee, infusion, and coffee grounds) by using a DI-SPME approach followed by GC/MS analysis. The procedure is eco-friendly and does not require any further pre-treatment of the sample, except a simple water dilution. The performances of the developed analytical method in terms of sensitivity, reproducibility, and recovery have proved to be adequate for the applications. Toxicity/carcinogenicity was much higher in dark roasted coffees contaminated with benzo[a]pyrene, although its estimated concentration levels were comparable to its LOD.
Data Availability
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
References
Aresta A, Cicco SR, Vona D, Farinola GM, Zambonin C (2022) Mussel inspired polydopamine as silica fibers coating for solid-phase microextraction. Separations 9:194–209
Binello A, Cravotto G, Menzio J, Tagliapietra S (2021) Polycyclic aromatic hydrocarbons in coffee samples: enquiry into processes and analytical methods. Food Chem 344:128631. https://doi.org/10.1016/j.foodchem.2020.128631
Choi H, Harrison R, Komulainen H, Delgado Saborit J.M. (2010) Polycyclic aromatic hydrocarbons. In: WHO guidelines for Indoor Air Quality: Selected Pollutants. Geneva, Switzerland: World Health Organization. 6. Available from: http://www.ncbi.nlm.nih.gov/books/NBK138709/
dos Santos RR, Vidotti Leal LD, de Lourdes CZ, Menezes HC (2019) Determination of polycyclic aromatic hydrocarbons and their nitrated and oxygenated derivatives in coffee brews using an efficient cold fiber-solid phase microextraction and gas chromatography mass spectrometry method. J Chromatogr A 1584:64–71. https://doi.org/10.1016/j.chroma.2018.11.046
Erdem P, Altınısik T, Seyhan Bozkurt S, Merdivan M (2021) Chitosan and dicationic ionic liquid intercalated clay-coated solid-phase microextraction fiber for determination of sixteen polycyclic aromatic hydrocarbons in coffee and tea samples. Talanta 235:122764
Gao P, da Silva E, Hou L, Denslow ND, Xiang P, Ma LQ (2018) Human exposure to polycyclic aromatic hydrocarbons: metabolomics perspective. Environ Int 119:466–477. https://doi.org/10.1016/j.envint.2018.07.017
Haritash AK, Kaushik CP (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169(1–3):1–15. https://doi.org/10.1016/j.jhazmat.2009.03.137
Houessou JK, Maloug S, Leveque A-S, Delteil C, Heyd B, Camel V (2007) Effect of roasting conditions on the polycyclic aromatic hydrocarbon content in ground arabica coffee and coffee brew. J Agric Food Chem 55(23):9719–9726
Huang YN, Chen J, Li Z, Wang L, Guan M, Qiu HD (2019) Porous graphene-coated stainless-steel fiber for direct immersion solid-phase microextraction of polycyclic aromatic hydrocarbons. Anal Methods 11:213–218
Jalili V, Barkhordari A, Ghiasvand A (2020) Solid-phase microextraction technique for sampling and preconcentration of polycyclic aromatic hydrocarbons: a review. Microchem J 157:104967. https://doi.org/10.1016/j.microc.2020.104967
Jimenez A, Adisa A, Woodham C, Salehj M (2014) Determination of polycyclic aromatic hydrocarbons in roasted coffee. Environ Sci Health B 49(11):828–835. https://doi.org/10.1080/03601234.2014.938552)
Johnsen AR, Wick LY, Harms H (2005) Principles of microbial PAH-degradation in soil. Environ Pollut 133(1):71–84. https://doi.org/10.1016/j.envpol.2004.04.015
Locatelli M, Forcucci L, Sciascia F, Cifelli R, Ferrone V, Carlucci G (2014) Extraction and detection techniques for PAHs determination in beverages: a review. Curr Chromatogr 1:122–138. https://doi.org/10.2174/2213240601666140415223545
Naccarato A, Gionfriddo E, Elliani R, Pawliszyn J, Sindona G, Tagarelli A (2018) Investigating the robustness and extraction performance of a matrix compatible solid-phase microextraction coating in human urine and its application to assess 2e6-ring polycyclic aromatic hydrocarbons using GCeMS/MS. J Separ Sci 41:929–939. https://doi.org/10.1002/jssc.201700989
Nisbet C, LaGoy P (1992) Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul Toxicol Pharm 16:290–300
Orecchio S, Paradiso Ciotti V, Culotta L (2009) Polycyclic aromatic hydrocarbons (PAHs) in coffee brew samples: analytical method by GC–MS, profile, levels and sources. Food Chem Toxicol 47(4):819–826
Pacheco-Fernandez I, Rentero M, Ayala JH, Pasan PV (2020) Green solid-phase microextraction fiber coating based on the metalorganic framework CIM-80(Al): analytical performance evaluation indirect immersion and headspace using gas chromatography and mass spectrometry for the analysis of water, urine and brewed coffee. Anal Chim Acta 1133:137–149
Pawliszyn J (2012) Theory of solid-phase microextraction, Elsevier Inc. https://doi.org/10.1016/B978-0-12-416017-0.00002-4
Purcaro G, Moret S, Conte LS (2013) Overview on polycyclic aromatic hydrocarbons: occurrence, legislation and innovative determination in foods. Talanta 105:292–305. https://doi.org/10.1016/j.talanta.2012.10.041
Rey-Salgueiro L, García-Falcón MS, Martínez-Carballo E, Simal-Gándara J (2008) Effects of toasting procedures on the levels of polycyclic aromatic hydrocarbons in toasted bread. Food Chem 108(2):607–615. https://doi.org/10.1016/j.foodchem.2007.11.026
Vas G, Vekey K (2004) Solid-phase microextraction: a powerful sample preparation tool prior to mass spectrometric analysis. J Mass Spectrom 39:233–254
Yazdi MN, Yamini Y, Asiabi H (2018) Multiwall carbon nanotube-zirconium oxide nanocomposite hollow fiber solid phase microextraction for determination of polyaromatic hydrocarbons in water, coffee and tea samples. J Chromatogr A 1554:8–15
Zambonin C, Aresta A (2021) Review: recent applications of solid phase microextractio coupled to liquid chromatography. Separations 8:34–48. https://doi.org/10.3390/separations8030034
Zelinkova Z, Wenzl T (2015) The occurrence of 16 EPA PAHs in food-a review. Polycycl Aromat Comp 35(2–4):248–284
Funding
Open access funding provided by Università degli Studi di Bari Aldo Moro within the CRUI-CARE Agreement. This work was supported by University of Bari “Aldo Moro”, Bari, Italy.
Author information
Authors and Affiliations
Contributions
A.M. Aresta and C. Zambonin conceived, designed the experiments, analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Human and Animal Rights
This study does not involve human participants, personal data or negative environmental impact.
Competing Interests
A.M. Aresta declares no competing interests. C. Zambonin declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aresta, A.M., Zambonin, C. Determination of Polycyclic Aromatic Hydrocarbons (PAHs) in Coffee Samples by DI-SPME-GC/MS. Food Anal. Methods 16, 1009–1016 (2023). https://doi.org/10.1007/s12161-023-02463-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-023-02463-y