Abstract
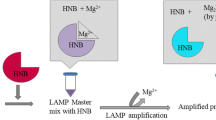
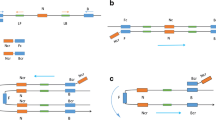
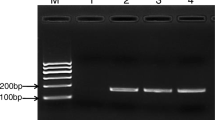
As a typical halophilic bacterium, Vibrio parahaemolyticus (V. parahaemolyticus) is regarded as one of the marine seafood-borne pathogens causing severe illnesses in humans and aquatic animals. Herein, a novel single primer isothermal amplification (SPIA) technique which was modified with SYBR Green II was successfully developed for the visual detection of V. parahaemolyticus. Interestingly, the visual superiority of SPIA can be achieved by fluorescence or precipitate to analyze the target pathogens in the seafood samples. The experimental results showed that all of V. parahaemolyticus strains were positive by using the novel SPIA assay, whereas the non-V. parahaemolyticus counterparts were negative. Furthermore, the limit of detection (LOD) of the SPIA assay was 5.7 CFU/mL in pure culture and 42 CFU/g in raw oysters by the visual fluorescence, respectively. Compared with the standard method, the developed SPIA approach achieved the satisfactory results with the characteristics of 100% sensitivity, 98.21% specificity, and 99.17% accuracy in the detection of 241 seafood samples. The developed SPIA method has been demonstrated as a rapid, specific, sensitive, and visual tool for the detection of V. parahaemolyticus, which could be adopted for routine surveillance of food-borne pathogens in the seafood samples.



Similar content being viewed by others
References
CFDA (2013) GB4789.7-2013. National food safety standard-Food microbiological examination-Examination of Vibrio parahaemolyticus. China Food and Drug Administration, Beijing, China
Chen L, Qiu Y, Tang H, Hu LF, Yang WH, Zhu XJ, Huang XX, Wang T, Zhang YQ (2018) ToxR Is Required for Biofilm Formation and Motility of Vibrio Parahaemolyticus. Biomed Environ Sci 31(11):848–850
Giuffrida MC, Spoto G (2017) Integration of isothermal amplification methods in microfluidic devices: Recent advances. Biosens Bioelectron 90:174–186
Han F, Ge B (2010) Quantitative detection of Vibrio vulnificus in raw oysters by real-time loop-mediated isothermal amplification. Int J Food Microbiol 142(1-2):60–66
Hossain MT, Kim YO, Kong IS (2013) Multiplex PCR for the detection and differentiation of Vibrio parahaemolyticus strains using the groEL, tdh and trh genes. Mol Cell Probes 27(5-6):171–175
ISO (2007) Microbiology of food and animal feeding stuffs- Horizontal method for the detection of potentially enteropathogenic Vibrio spp. -- Part 1: Detection of Vibrio parahaemolyticus and Vibrio cholerae (ISO/TS 21872-1:2007). International Organization for Standardization (ISO), Geneva
Jiang Y, Chu Y, Xie G, Li F, Wang L, Huang J, Zhai Y, Yao L (2019) Antimicrobial resistance, virulence and genetic relationship of Vibrio parahaemolyticus in seafood from coasts of Bohai Sea and Yellow Sea, China. Int J Food Microbiol 290:116–124
Jones JL, Ludeke CH, Bowers JC, Garrett N, Fischer M, Parsons MB, Bopp CA, DePaola A (2012) Biochemical, serological, and virulence characterization of clinical and oyster Vibrio parahaemolyticus isolates. J Clin Microbiol 50(7):2343–2352
Jones JL, Ludeke CH, Bowers JC, DeRosia-Banick K, Carey DH, Hastback W (2014) Abundance of Vibrio cholerae, V. vulnificus, and V. parahaemolyticus in oysters (Crassostrea virginica) and clams (Mercenaria mercenaria) from Long Island sound. Appl Environ Microbiol 80(24):7667–7672
Kirs M, Depaola A, Fyfe R, Jones JL, Krantz J, Van Laanen A, Cotton D, Castle M (2011) A survey of oysters (Crassostrea gigas) in New Zealand for Vibrio parahaemolyticus and Vibrio vulnificus. Int J Food Microbiol 147(2):149–153
Kumar BK, Raghunath P, Devegowda D, Deekshit VK, Venugopal MN, Karunasagar I, Karunasagar I (2011) Development of monoclonal antibody based sandwich ELISA for the rapid detection of pathogenic Vibrio parahaemolyticus in seafood. Int J Food Microbiol 145(1):244–249
Kurn N, Chen P, Heath JD, Kopf-Sill A, Stephens KM, Wang S (2005) Novel isothermal, linear nucleic acid amplification systems for highly multiplexed applications. Clin Chem 51(10):1973–1981
Lei T, Jiang F, He M, Zhang J, Zeng H, Chen M, Pang R, Wu S, Wei L, Wang J, Ding Y, Wu Q (2020) Prevalence, virulence, antimicrobial resistance, and molecular characterization of fluoroquinolone resistance of Vibrio parahaemolyticus from different types of food samples in China. Int J Food Microbiol 317:108461
Ling N, Shen J, Guo J, Zeng D, Ren J, Sun L, Jiang Y, Xue F, Dai J, Li B (2020) Rapid and accurate detection of viable Vibrio parahaemolyticus by sodium deoxycholate-propidium monoazide-qPCR in shrimp. Food Control 109:106883
Liu N, Zou D, Dong D, Yang Z, Ao D, Liu W, Huang L (2017) Development of a multiplex loop-mediated isothermal amplification method for the simultaneous detection of Salmonella spp. and Vibrio parahaemolyticus. Sci Rep 7:45601
Rosec JP, Simon M, Causse V, Boudjemaa M (2009) Detection of total and pathogenic Vibrio parahaemolyticus in shellfish: comparison of PCR protocols using pR72H or toxR targets with a culture method. Int J Food Microbiol 129(2):136–145
Shen M, Duan N, Wu S, Zou Y, Wang Z (2018) Polydimethylsiloxane gold nanoparticle composite film as structure for aptamer-based detection of Vibrio parahaemolyticus by surface-enhanced raman spectroscopy. Food Anal Methods 12(2):595–603
Sobrinho Pde S, Destro MT, Franco BD, Landgraf M (2010) Correlation between environmental factors and prevalence of Vibrio parahaemolyticus in oysters harvested in the southern coastal area of Sao Paulo State, Brazil. Appl Environ Microbiol 76(4):1290–1293
Suleman E, Mtshali MS, Lane E (2016) Investigation of false positives associated with loop-mediated isothermal amplification assays for detection of Toxoplasma gondii in archived tissue samples of captive felids. J Vet Diagn Investig 28(5):536–542
Taiwo M, Baker-Austin C, Powell A, Hodgson E, Natås OB, Walker DI (2017) Comparison of toxR and tlh based PCR assays for Vibrio parahaemolyticus. Food Control 77:116–120
Wang R, Zhong Y, Gu X, Yuan J, Saeed AF, Wang S (2015) The pathogenesis, detection, and prevention of Vibrio parahaemolyticus. Front Microbiol 6:144
Wang Z, Yang Q, Zhang Y, Meng Z, Ma X, Zhang W (2017) Saltatory rolling circle amplification (SRCA): a novel nucleic acid isothermal amplification technique applied for rapid detection of Shigella spp. in vegetable salad. Food Anal Methods 11(2):504–513
Whitaker WB, Parent MA, Boyd A, Richards GP, Boyd EF (2012) The Vibrio parahaemolyticus ToxRS regulator is required for stress tolerance and colonization in a novel orogastric streptomycin-induced adult murine model. Infect Immun 80(5):1834–1845
Wu Z (2019) A dual-mode (fluorometric and colorimetric) aptasensor for Vibrio parahaemolyticus detection using multifunctional nanoparticles. Food Anal Methods 12(7):1577–1584
Yamazaki W, Ishibashi M, Kawahara R, Inoue K (2008) Development of a loop-mediated isothermal amplification assay for sensitive and rapid detection of Vibrio parahaemolyticus. BMC Microbiol 8(1):163
Yan W, Zhang J, Rui Z, Sun Y, Jie R, Ding X (2015) Combination of SYBR Green II and TaqMan probe in the adulteration detection of Dendrobium devonianum by fluorescent quantitative PCR. Int J Food Sci Technol 50(12):2572–2578
Zhang Z, Xiao L, Lou Y, Jin M, Liao C, Malakar PK, Pan Y, Zhao Y (2015) Development of a multiplex real-time PCR method for simultaneous detection of Vibrio parahaemolyticus, Listeria monocytogenes and Salmonella spp. in raw shrimp. Food Control 51:31–36
Zhang Y, Gao H, Osei-Adjei G, Zhang Y, Yang W, Yang H, Yin Z, Huang X, Zhou D (2017) Transcriptional regulation of the type VI secretion system 1 genes by quorum sensing and ToxR in Vibrio parahaemolyticus. Front Microbiol 8:2005
Zhang M, Wu P, Wu J, Ping J, Wu J (2019) Advanced DNA-based methods for the detection of peanut allergens in processed food. TrAC Trends Anal Chem 114:278–292
Zhang M, Liu C, Shi Y, Wu J, Wu J, Chen H (2020) Selective endpoint visualized detection of Vibrio parahaemolyticus with CRISPR/Cas12a assisted PCR using thermal cycler for on-site application. Talanta 214:120818
Zhong Q, Tian J, Wang B, Wang L (2016) PMA based real-time fluorescent LAMP for detection of Vibrio parahaemolyticus in viable but nonculturable state. Food Control 63:230–238
Zhu P, Gao W, Huang H, Jiang J, Chen X, Fan J, Yan X (2018) Rapid detection of Vibrio parahaemolyticus in shellfish by real-time recombinase polymerase amplification. Food Anal Methods 11(8):2076–2084
Funding
This study was supported by the National Natural Science Foundation of China (31371772), the Natural Science Foundation of Hebei Province (C2019204342), the Scientific Research Program of Hebei Education Department (ZD2017237), the Key Research and Development Project of Hebei Province of China (18275501D), the Food Processing Discipline Group of Hebei Agricultural University (No. 2021-06) and the Program of Introduced Doctor of Hebei Agricultural University (ZD201623).
Author information
Authors and Affiliations
Contributions
Qian Yang, Wei Guo, and Yi Liu contributed equally to this work. Qian Yang and Ruoyang Ming developed methodology and performed data acquisition. Wei Guo contributed to the conception, revision, and editing. Yi Liu and Yunzhe Zhang contributed to the methodology and analysis. Wei Zhang, Yaowu Yuan, and Jianxin Tan contributed to the study conception and design. The draft of the manuscript was written by Qian Yang. Wei Zhang made critical corrections. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 2933 kb)
Rights and permissions
About this article
Cite this article
Yang, Q., Guo, W., Liu, Y. et al. Novel Single Primer Isothermal Amplification Method for the Visual Detection of Vibrio parahaemolyticus. Food Anal. Methods 14, 1995–2002 (2021). https://doi.org/10.1007/s12161-021-02033-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-021-02033-0