Abstract
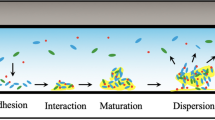
Biofilms are organized bacterial communities embedded in extracellular polymeric matrices attached to living or abiotic surfaces. Eradicating biofilms completely is challenging and leads to repeated pollution. Thus, development of on-site and rapid biofilm detection methods is necessary. Various approaches for measuring biofilm formation have been implemented over the last decade. These include the crystal violet (CV) assay and confocal laser scanning microscopy (CLSM). However, the existing approaches are associated with issues, such as complexity and length of analysis. Consequently, in this work, a novel and rapid detection technique, specifically surface-enhanced Raman scattering (SERS), was investigated for the measurement of the Staphylococcus aureus biofilm formation. After 5, 7, 9, 11, and 13 days of culture in simulated pipes, the biofilm growth was analyzed by SERS, CV assay, and CLSM. The study revealed that the SERS approach gave optimal results following linear fitting at the relative peak intensities of I1172 cm–1/I722 cm–1 and I1384 cm–1/I722 cm–1, with the R2 values of 0.9576 and 0.9640. It was established that the two characteristic Raman peaks reflected the change of biofilm more adequately. The SERS method was compared with the CV assay and CLSM by Pearson correlation analysis. In addition to a good correlation between SERS and CV assay (P < 0.05), a significant correlation between SERS and CLSM (P < 0.01) was determined. Moreover, the novel SERS method considerably shortened the detection time, eliminating the necessity for an elution step. Overall, the described technique is a reliable on-site and non-destructive biofilm formation detection method.






Similar content being viewed by others
References
Albrecht MG, Creighton JA (2002) Anomalously intense Raman spectra of pyridine at a silver electrode. J Am Chem Soc99:5215–5217
Bridier A, Dubois-Brissonnet F, Boubetra A, Thomas V, Briandet R (2010) The biofilm architecture of sixty opportunistic pathogens deciphered using a high throughput CLSM method. J Microbiol Methods82:64–70
Cardinal MF, Ende EV, Hackler RA, McAnally MO, Stair PC, Schatz GC, Van Duyne RP (2017) Expanding applications of SERS through versatile nanomaterials engineering. Chem Soc Rev46:3886–3903
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science284:1318–1322
Cuong Quoc N, Thrift WJ, Bhattacharjee A, Ranjbar S, Gallagher T, Darvishzadeh-Varcheie M, Sanderson RN, Capolino F, Whiteson K, Baldi P, Hochbaum AI, Ragan R (2018) Longitudinal monitoring of biofilm formation via robust surface enhanced Raman scattering quantification of Pseudomonas aeruginosa-produced metabolites. ACS Appl Mater Interfaces10:12364–12373
Dasary SSR, Singh AK, Senapati D, Yu H, Ray PC (2009) Gold nanoparticle based label-free SERS probe for ultrasensitive and selective detection of trinitrotoluene. J Am Chem Soc131:13806–13812
Fan W, Lee YH, Pedireddy S, Zhang Q, Liu T, Ling XY (2014) Graphene oxide and shape-controlled silver nanoparticle hybrids for ultrasensitive single-particle surface-enhanced Raman scattering (SERS) sensing. Nanoscale6:4843–4851
Fleischmann M, Hendra PJ, McQuillan AJ (1974) Raman spectra of pyridine adsorbed at a silver electrode. Chem Phys Lett26:163–166
Flemming HC, Wingender J (2001) Relevance of microbial extracellular polymeric substances (EPSs) - Part I: Structural and ecological aspects. Water Sci Technol43:1–8
Foster TJ, Geoghegan JA, Ganesh VK, Hoeoek M (2014) Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol12:49–62
Jeanmaire DL, Richard P (1977) Surface Raman spectroelectrochemistry. Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J Electroanal Chem84:1–20
Kahraman M, Mullen ER, Korkmaz A, Wachsmann-Hogiu S (2017) Fundamentals and applications of SERS-based bioanalytical sensing. Nanophotonics6:831–852
Kleinman SL, Ringe E, Valley N, Wustholz KL, Phillips E, Scheidt KA, Schatz GC, Van Duyne RP (2011) Single-molecule surface-enhanced Raman spectroscopy of crystal violet isotopologues: theory and experiment. J Am Chem Soc133:4115–4122
Kniggendorf AK, Meinhardt-Wollweber M (2011) Of microparticles and bacteria identification - (resonance) Raman micro-spectroscopy as a tool for biofilm analysis. Water Res45:4571–4582
Kniggendorf AK, Nogueira R, Kelb C, Schadzek P, Meinhardt-Wollweber M, Ngezahayo A, Roth B (2016) Confocal Raman microscopy and fluorescent in situ hybridization - a complementary approach for biofilm analysis. Chemosphere161:112–118
Kumar CG, Anand SK (1998) Significance of microbial biofilms in food industry: a review. Int J Food Microbiol42:9–27
Lopez-Diez EC, Winder CL, Ashton L, Currie F, Goodacre R (2005) Monitoring the mode of action of antibiotics using Raman spectroscopy: investigating subinhibitory effects of amikacin on Pseudomonas aeruginosa. Anal Chem77:2901–2906
Marks H, Schechinger M, Garza J, Locke A, Cote G (2017) Surface enhanced Raman spectroscopy (SERS) for in vitro diagnostic testing at the point of care. Nanophotonics6:681–701
Morillas H, Maguregui M, Marcaida I, Trebolazabala J, Salcedo I, Madariaga JM (2015) Characterization of the main colonizer and biogenic pigments present in the red biofilm from La Galea Fortress sandstone by means of microscopic observations and Raman imaging. Microchem J121:48–55
O’Toole GA (2011) Microtiter dish biofilm formation assay. Journal of Visualized Experiments, Microtiter Dish Biofilm Formation Assay
Pätzold R, Keuntje M, Ahlften AA (2006) A new approach to non-destructive analysis of biofilms by confocal Raman microscopy. Anal Bioanal Chem386:286–292
Pätzold R, Keuntje M, Theophile K, Mueller J, Mielcarek E, Ngezahayo A, Ahlften AA (2008) In situ mapping of nitrifiers and anammox bacteria in microbial aggregates by means of confocal resonance Raman microscopy. J Microbiol Methods72:241–248
Peron O, Rinnert E, Lehaitre M, Crassous P, Compere C (2009) Detection of polycyclic aromatic hydrocarbon (PAH) compounds in artificial sea-water using surface-enhanced Raman scattering (SERS). Talanta79:199–204
Pradhan N, Pradhan SK, Nayak BB, Mukherjee PS, Sukla LB, Mishra BK (2008) Micro-Raman analysis and AFM imaging of Acidithiobacillus ferrooxidans biofilm grown on uranium ore. Res Microbiol159:557–561
Rebrosova K, Siler M, Samek O, Ruzicka F, Bernatova S, Jezek J, Zemanek P, Hola V (2017) Differentiation between Staphylococcus aureus and Staphylococcus epidermidis strains using Raman spectroscopy. Future Microbiol12:881–890
Silge A, Schumacher W, Roesch P, Da Costa Filho PA, Gerard C, Popp J (2014) Identification of water-conditioned Pseudomonas aeruginosa by Raman microspectroscopy on a single cell level. Syst Appl Microbiol37:360–367
Suci PA, Geesey GG, Tyler BJ (2001) Integration of Raman microscopy, differential interference contrast microscopy, and attenuated total reflection Fourier transform infrared spectroscopy to investigate chlorhexidine spatial and temporal distribution in Candida albicans biofilms. J Microbiol Methods46:193–208
Sun P, Hui C, Wang S, Wan L, Zhang X, Zhao Y (2016) Bacillus amyloliquefaciens biofilm as a novel biosorbent for the removal of crystal violet from solution. Colloids Surf B-Biointerf139:164–170
Vogel H, Jahnig F (1986) Models for the structure of outer-membrane proteins of Escherichia coli derived from Raman spectroscopy and prediction methods. J Mol Biol190:191–199
Wang J, Wu X, Wang C, Shao N, Dong P, Xiao R, Wang S (2015) Magnetically assisted surface-enhanced Raman spectroscopy for the detection of Staphylococcus aureus based on aptamer recognition. ACS Appl Mater Interfaces7:20919–20929
Wells AV, Li PS, Champion PM, Martinis SA, Sligar SG (1992) Resonance Raman investigations of Escherichia-coli-expressed Pseudomonas-putida cytochrome-P450 and cytochrome-P420. Biochemistry31:4384–4393
Yerly J, Hu Y, Jones SM, Martinuzzi RJ (2007) A two-step procedure for automatic and accurate segmentation of volumetric CLSM biofilm images. J Microbiol Methods70:424–433
Funding
The following founding sources are gratefully acknowledged: National Key R&D Program of China (2018YFC1602300), Natural Science Foundation of Jiangsu Province (No. BK20171139), China Postdoctoral Science Foundation funded project (2018 M642165), Yangtze River Delta Project of Shanghai (18395810200), National first-class discipline program of Food Science and Technology (JUFSTR20180509).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Liu Yang declares that he has no conflict of interest. Hang Yu declares that he has no conflict of interest. Yuliang Cheng declares that he has no conflict of interest. Yahui Guo declares that he has no conflict of interest. Weirong Yao declares that he has no conflict of interest. Yunfei Xie declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Yu, H., Cheng, Y. et al. Non-destructive Monitoring of Staphylococcus aureus Biofilm by Surface-Enhanced Raman Scattering Spectroscopy. Food Anal. Methods 13, 1710–1716 (2020). https://doi.org/10.1007/s12161-020-01792-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-020-01792-6