Abstract
Extraction of antioxidants from bilberries using solvent extraction and the novel rotating bed reactor (RBR) both with one-phase (ethanol and water) and two-phase (ethanol and water +salt) solvents was studied. Solids, ethanol, and temperature settings in an experimental design were monitored for 1 h. The measured responses were (1) polyphenol concentration, (2) visible-near infrared spectra, and (3) HPLC measurement. The (1) responses were used for making response surfaces in time and the spectra (2) could confirm these results. The HPLC results (3) confirmed the results of 1 and 2 but were found unsuitable for online monitoring. The RBR was better than traditional extraction and 16 min sufficed. The response surfaces showed an optimal concentration of ethanol, temperatures above 50 °C gave the best results, and high loads of solid were beneficial. Two-phase extraction was less efficient. The methodology could be transferred to larger scale extraction systems to improve yield and save on reagents/energy cost.
Similar content being viewed by others
Introduction
Berries and Bilberries
Berries from the Nordic forests are very popular on the world market (Zoratti et al. 2015) because their extracts have been used as food and health supplements (Basu et al. 2010; Pascu et al. 2013) as well as functional ingredients in cosmetics production by a number of industries (Benzie and Wachtel-Galor 2011; Moze et al. 2011).
Polyphenolic substances from berries and other fruits and vegetables are soluble in blood and able to cross the blood-brain barrier. They are assumed to serve as antioxidants and thereby to avert diseases (Olas 2018).
Anthocyanidins
The color in berries originates from anthocyanidins which are polyphenols. These polyphenols are important pigments causing the color of fruit and berries in a low pH decompose them quickly.
Anthocyanidins are important constituents present in berry peels and juices while the berry seeds contain oils that also are useful for health and cosmetic products. The content of anthocyanidin in berries increases during the growth season and is optimal during late summer.
Bilberries have been demonstrated as important resources for anthocyanidin (550–850 mg/100 g) (Heffels et al. 2015; Hidalgo and Almajano 2017; Maatta-Riihinen et al. 2004).
Around 5–10% of the bilberries (Vaccinium myrtillus) grown in Swedish forests are picked for private and commercial use (Paassilta et al. 2009). A short explanation of terms is needed here: anthocyanidins are a group of molecules based on the same flavonoids structure, but with different substituents in seven locations of the molecule. These substituents are H, OH, and OCH3 as seen in Fig. 1. For this reason, anthocyanidins are also called polyphenols. Anthocyanins are anthocyanidins bound to sugars that occur in many plants (Maatta-Riihinen et al. 2004).
Press Cakes
Bilberries are used industrially to make fruit juices and jams. During the production of the juice, a press cake is left that contains a large amount of anthocyanidins (Aaby et al. 2013; Dinkova et al. 2014; Nour et al. 2015). The antioxidants can be extracted from press cakes with a water-ethanol mixture and the efficiency of extraction is affected by ethanol concentration, amount of powder, temperature, and time (Iglesias-Carres et al. 2018; Klavins et al. 2018). The extraction settings can be varied and this should be done according to an experimental design where the settings are used as factors. For industrial applications, both a high concentration and a short extraction time are important so these aspects of the extraction have to be tested in order to find a reasonable compromise.
Extraction and Concentration Measurement
The two novelties introduced in this paper are RBR extraction and two-phase extraction. These are compared to each other and to classical extraction. Others have also used microwave extraction or supercritical CO2 which we did not have available (Babova et al. 2016; Eliasson et al. 2017; Fidale et al. 2016; Hidalgo and Almajano 2017).
The three extraction setups tested were traditional solvent extraction (TSE) and the novel rotating bed reactor (RBR) both with one-phase and two-phase solvents (Grilo et al. 2016). The laboratory extractions were carried out as repeated experimental designs. As response variable for the designs, three alternatives were proposed by us: Folin-Ciocalteu (FC) reagent followed by spectroscopic measurement of the resulting blue color, VIS-NIR spectroscopy by transmission through the extract, and HPLC analysis for determining single anthocyanidin concentrations. The aims were (1) to compare the three extraction methods, (2) to find optimum setting for the extractions, and (3) to test the three monitoring techniques.
Data Analysis
All experiments were carried out as experimental designs and multivariate regression coefficients were calculated and interpreted. Response surface methodology was used to check for optimal regions of the parameter settings. The spectroscopic data were analyzed multivariately by making regression models.
Materials and Methods
Bilberry Press Cake
Press cake was acquired from a commercial bilberry juice producer in northern Sweden. A randomly chosen unpretreated press cake of 8 kg was freeze dried (Edwards E2M5 − 50 °C and 8 hPa) and milled (Retsch Mixer mill MM 400) to produce a homogeneous powder that was kept frozen until use.
Experimental Design
The three factors used for the experimental design were total solids (TS), mass % of ethanol used, and temperature. Temperature and % ethanol are expected to influence the extraction. The amount of extracted polyphenols should increase as the TS goes up but it was important to check this factor for nonlinearities. The extraction experimental design for methods 1 and 2 was based on a slightly modified three-factor Box-Behnken (BB) design with 12 runs (Myers and Montgomery 2002). To this design, 6 star points were added and the center point was replicated 3 times, resulting in 21 runs in total. A central composite circumscribed (CCC) design with 12 runs was used for the two-phase experiments (methods 3 and 4).
The TSE used double the solvent volume of the RBR so the TS levels were also doubled. Extraction time is also a factor but it was not included in the models. Instead, each run was checked over time: TSE for 0, 8, 16, 32, and 64 min and RBR for 2, 4, 8, 16, 32, and 64 min. This way each of the times has its own complete design available. Times were chosen for practical reasons. RBR allowed measurement after 2 and 4 min while for TSE, this could not be done.
The levels used for ethanol were in mass %: star low = 10, low = 20, center = 40, high = 60, and star high = 70. For temperature (°C), the star points were chosen inside the BB design for practical reasons in star low = 30, low = 20, center = 40, high = 60, and star high = 50. For the RBR-dried press cake (g) used for extraction, the levels were star low = 1.05, low = 3.33, center = 5, high = 10, and star high = 12.27 while for the classical extraction; these masses were 2.11 g, 6.66 g, 10 g, 20 g, and 24.5 g (approximately double those for RBR). This is a deviation from the ideal textbook values, but still possible to use for calculations.
Extraction of Anthocyanidins
Traditional Solvent Extraction (Method 1)
The solvent mass used was 400 g each time, with ethanol amounts of 40 g, 80 g, 160 g, 240 g, and 280 g and the remaining part to a total weight of 400 g was filled with distilled water. The different design temperatures were maintained by using a climate chamber. Magnetic stirring at 300 rpm was used. After temperature equilibration in the climate chamber, the press cake powder was added. Ten-milliliter samples were taken out and filtered after 0, 8, 16, 32, and 64 min; these were used for FC and NIR measurement. After 64 min, two 15-mL samples were taken out and sent for HPLC analysis (Fig. 2).
Rotating Bed Reactor (Method 2)
In the RBR reactor (SpinChem), the solid is added to and contained in a rotating drum and the solvent circulates through a chamber due to the centrifugal forces created by the rotation. Figure 3 gives a schematic of the flows and the working principle of the RBR reactor. The RBR can be temperature controlled by pumping water from a thermostatic bath though the jacket around the chamber. All pumping was done with peristaltic pumps. Sampling was done after 2, 4, 8, 16, 32, and 64 min for NIR and FC measurement. Final samples taken after 64 min were analyzed by HPLC.
Two-Phase Extraction (Method 3 and 4)
A special technique tested in the present paper is two-phase extraction where salt solution/ethanol mixtures generate a two-phase system when high concentrations of certain salts are added. The two-phase system has an additional advantage that polyphenols end up in the ethanol phase while sugars end up in the water phase which did not display color. With many other extraction techniques, the sugars remain mixed with the anthocyanidins.
The system of two-phase extraction was tested with two different salts namely NaH2PO4 and (NH4)2SO4 (respectively methods 3 and 4). For each salt, an experimental design in the factors TS and temperature was carried out. The extractions were carried out over time using the intervals 2, 4, 8, 16, 32, and 64 min.
Method 3 used: 96 g H2O, 54 g Na2HPO4 × 2 H2O (VWR, Analar), and 50 g EtOH.
Method 4 used: 102 g H2O, 38 g (NH4)2SO4 (VWR Analar), and 60 g EtOH.
To summarize, the compared extraction methods were as follows:
- 1.
Traditional extraction using different concentrations of ethanol dissolved in water, different masses of material, and different temperatures.
- 2.
RBR extraction using different concentrations of ethanol dissolved in water, different masses, and temperatures.
- 3.
RBR extraction using two-phase system, method 3 only mass and temperature were changed.
- 4.
RBR extraction using two-phase system method 4, only mass and temperature were changed.
Analysis of Total Phenolics and Anthocyanidins
Total Phenolic Content
The Folin-Ciocalteu (FC) reagent (Sigma Aldrich) is a mixture of tungstenate/molybdate/phosphoric acid reagent in hydrochloric acid that acts as a colorimetric assay for polyphenolic antioxidants, proteins, and some other analytes. The recipe used was one optimized for polyphenols. The intensity of the blue color is related to the amount of polyphenols present in solution. The blue color was measured in a transmission cuvette at 765 nm (Singleton et al. 1999). A calibration curve was made using gallic acid (Sigma). Transmission measurement was done using a Shimadzu UV-1800 (Shimadzu Corp, Japan).
NIR Spectroscopy
Visible and NIR spectroscopy can be used to identify and quantify many colored and other organic chemicals (Burns and Ciurczak 2001; Siesler et al. 2007). The extract was measured by a Tec5 VIS-NIR spectrometer (Tec5, Germany) using a homebuilt transmission flow cell with 5-mm transmission path length. The liquid was pumped through the flow cell. The Tec5 gives responses at wavelengths 305–2200 nm, but the ranges above 1400 nm were omitted because it mainly represented noise and irrelevant information, see Fig. 2. The Agrospec software was used to control the Tec5.
Anthocyanidin Measurement by HPLC
The content of individual anthocyanidins, delphinidin, cyanidin, petunidin, pelargonidin, peonidin, and malvidin, was measured by HPLC in the literature (Swadseh 1996). Our instrument was a Shimadzu LC-20A Prominence equipped with an autosampler. Eluent used for analysis was (A) 0.4% TFA in water and (B) 0.4% TFA in acetonitrile. Gradient program was as follows: 0–4 min, 84% A; 4–15 min, 84–62% A; 15–16 min 62% A; 16–16.1 min 84% A; 16.1–17 min 84% A, stop. Analytical separation of anthocyanidins was carried out using a Poroshell 120 SB-C18 (4.6 × 75 mm; 2.7 μm) column. The column oven was set at 30 °C and the flow of the mobile phase was 1.0 mL/min. Anthocyanidins were detected at 530 nm using a photodiode array 190–800 nm.
All the standards (delphinidin, cyanidin, petunidin, pelargonidin, peonidin, and malvidin chloride) were purchased from Extrasynthese. Water was ultrapure quality water (Millipore) and acetonitrile was of HPLC grade. Stock solution for each individual standard was 5 mg/mL. Working standards were diluted as follows: 62.5, 125, 250, and 500 μg/mL where each standard contains all six individual anthocyanidins. Standards were used to generate a four-point calibration curve. Identification was based on retention time and UV-spectra of the single standard.
Multivariate Data Analysis
Much emphasis in the paper is on data analysis, both analyzing the experimental designs and analyzing the multivariate spectroscopic data. All the experiments were carried out according to experimental designs. For analysis of a design, multivariate linear regression (MLR) is used for modeling responses from experimental settings and their transformed values (Box and Draper 1987). The designs were subjected to a complete analysis by regression coefficients and determination of their significance. Response surface figures were made for some designs. The experimental designs were analyzed in the Modde 10.1 software (Umetrics, Sartorius Stedim Biotech, Sweden). For the spectroscopic analysis by VIS-NIR, multivariate analysis by PCA and PLS was used. Evince software (Prediktera, Sweden) was used for this purpose.
Results and Discussion
Experimental Design
With the FC polyphenol results as responses, the previously described designs can be analyzed. The analysis was done by multiple linear regression (MLR) for each of the time points. Table 1 gives some of the diagnostics for the different designs.
For the RBR case, the R2 and Q2 show low values after 2 and 4 min and begin to stabilize after 8 min. The predicted maximum increases as a function of time. TSE has high R2 and Q2 for all sampling times. The predicted maximum is higher than for RBR for 0 to 8 min but after 16 min, the RBR has a higher yield maximum. This is due to slower diffusion of the liquid in the solid phase. For the two-phase case, the salt mixture used in method 3 was not stable (precipitation occurred) and this led to unreliable results. Method 3 was therefore left out of the data analysis. Method 4 gave high concentrations in the ethanol phase, but here the volume was less, so the total obtained amount was not as high as for methods 1 and 2.
R2 is the coefficient of determination from MLR. Q2 is the same value obtained by cross-validation. The maximum obtainable value in the response surface was calculated using the MODDE program. According to Table 1, both the TSE and RBR extractions could be stopped after 16 min. The increase for 48 min extra is only 11% for TSE. For RBR, the polyphenol concentration is higher than for TSE after 16 min. For the RBR, the increase after 48 extra minutes is 21%.
The factor TS is very important and has a linear behavior while EtOH has a nonlinear behavior, see upper Fig. 4. The influence of temperature is not significant which is advantageous as high temperatures cause degradation of the anthocyanidins. The highest yield is obtained after 64 min, upper Fig. 5 shows the contour plots for the RBR extraction at 64 min, but the contour plots look similar for 16 min (data not shown).
The MLR coefficients for the TSE are shown in lower Fig. 4. TS is significant, but without nonlinear contribution. Ethanol has a nonlinear behavior, but also temperature has a significant influence. The response surface for 64 min seen in lower Fig. 5 shows a lesser maximum than that for the RBR extraction.
The TSE gave very similar coefficients for all times, so only the regression coefficient plot for 16 min extraction is shown in Fig. 4. It shows the great importance of TS but also some importance for temperature and a nonlinear behavior for ethanol. Therefore, the obtained response surfaces for different extraction time were almost identical in shape, but different in yield. Figure 5 shows the response surface after 64 min.
Multivariate Calibration of NIR Data
After omitting the noisy regions above 1400 nm, a wavelength range of 1096 wavelengths was left. This gives two matrices, 125 objects and 1096 variables for RBR and 105 objects and 1096 for TSE. VIS-NIR measurements are easy and fast to carry out and they are therefore ideal for online measurement. It is possible to pump the extraction solvent though a transmission cell and to get almost immediate results. If these results correspond to the polyphenol concentration, then NIR can be used to predict the polyphenol concentrations. In order to test this, a PLS model needs to be made using the polyphenol FC concentrations as dependent variables.
The results shown here are for PLS with polyphenol concentration FC as response variable. Two obvious outliers were removed for RBR. For RBR, a coefficient of determination of 0.70 was reached after three components. For the traditional method, a coefficient of determination of 0.82 was reached after three components and the increase with more components was very slow. Therefore, three components were deemed sufficient for both cases. Detailed PLS results are not shown, but the PLS scores were used for the PLS score plot in Fig. 6.
PLS score plots circles colored according to FC polyphenol concentration and size of the symbol indicate TS amount. Upper: RBR extraction, component 1 (R2Y = 35%) component 2 (R2Y = 17%). Middle: RBR extraction, component 1 (R2Y = 35%) component 3 (R2Y = 18%). Lower TSE extraction for component 1 (R2Y = 38%) and for component 2 (R2Y = 28%)
Both RBR score plots show that the interesting results with high extraction yield are in one region of the plot. For the TSE case, only one plot is shown. Also, in this plot, a clear region of high extraction yields is discernable. Plots like these could be used for monitoring purposes using PLS prediction from online measured spectra over time. The three figures clearly indicate the usefulness of experimental design also when looking at spectra.
HPLC
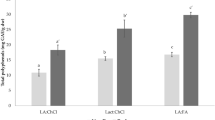
HPLC is the only way of getting concentrations for individual anthocyanidins, but the analyses are expensive and slow. For the RBR extraction, the correlation table between HPLC determined anthocyanidins and the FC polyphenols gave coefficients > 0.95 for all anthocyanidins, i.e., the analytical results were much correlated as expected. For the TSE, high correlation > 0.9 was obtained except for pelargonidin that had low correlation coefficients due to some concentrations being below detection limit. This shows that the FC determination is a representative method for measuring total polyphenol. The conclusion is also that there is no selective extraction of individual anthocyanidins but other authors have used pH differences for selective extraction (Wrolstad et al. 2005).
The averaged measured anthocyanidin concentration (delphinidin, cyanidin, petunidin, peonidin, and malvidin) indicates that RBR extraction gives a higher yield for all, not shown here. Pelargonidin was often below detection limit.
Conclusions
Of the four extractions tested, three worked well. RBR seemed to give similar yields than TSE but was more practical especially with the shorter extraction times. Sixty-four minutes was ideal for obtaining maximal yield, but in large-scale operations, the time aspect is of essence. The results indicate that a 16-min extraction could be cost-effective for RBR.
As response variables, the Folin reaction gave good results for total polyphenols, while the HPLC gave useful results for individual anthocyanidins but was much slower. The VIS-NIR measurement does not give direct polyphenol concentrations, but could be used to determine these by multivariate methods. The VIS-NIR measurement is the only analysis technique that is fast enough to be implemented online in industry. Because of the use of experimental design, it was possible to construct response surfaces showing the sometimes nonlinear effect of temperature, time, and ethanol concentration. It was also possible to use score plots from PLS regression on the VIS-NIR spectra to get similar conclusions as those of the response surface.
References
Aaby K, Grimmer S, Holtung L (2013) Extraction of phenolic compounds from bilberry (Vaccinium myrtillus L) press residue: effects on phenolic composition and cell proliferation. LWT-Food Sci Technol 54:257–264. https://doi.org/10.1016/j.lwt.2013.05.031
Babova O, Occhipinti A, Capuzzo A, Maffei ME (2016) Extraction of bilberry (Vaccinium myrtillus) antioxidants using supercritical/subcritical CO2 and ethanol as co-solvent. J Supercrit Fluids 107:358–363. https://doi.org/10.1016/j.supflu.2015.09.029
Basu A, Rhone M, Lyons TJ (2010) Berries: emerging impact on cardiovascular health. Nutr Rev 68:168–177. https://doi.org/10.1111/j.1753-4887.2010.00273.x
Benzie IFF, Wachtel-Galor S (2011) Herbal Medicine: biomolecular and clinical aspects. CRC Press/Taylor & Francis, Boca Raton
Box GEP, Draper NR (1987) Empirical model-building and response surfaces. Wiley, Hoboken
Burns DA, Ciurczak EW (2001) Handbook of Near-Infrared Analysis, 2nd edn. York, New
Dinkova R, Heffels P, Shikov V, Weber F, Schieber A, Mihalev K (2014) Effect of enzyme-assisted extraction on the chilled storage stability of bilberry (Vaccinium myrtillus L.) anthocyanins in skin extracts and freshly pressed juices. Food Res Int 65:35–41. https://doi.org/10.1016/j.foodres.2014.05.066
Eliasson L, Labrosse L, Ahrne L (2017) Effect of drying technique and particle size of bilberry press cake on the extraction efficiency of anthocyanins by pressurized carbon dioxide extraction. LWT-Food Sci Technol 85:510–516. https://doi.org/10.1016/j.lwt.2017.03.030
Fidale M, Lavecchia R, Zuorro A (2016) Extraction of bioactive polyphenols with high antioxidant activity from bilberry (Vaccinium myrtillus L.). Process Waste Orient J Chem 32:759–767. https://doi.org/10.13005/ojc/320201
Grilo AL, Aires-Barros MR, Azevedo AM (2016) Partitioning in aqueous two-phase systems: fundamentals, applications and trends. Sep Purif Rev 45:68–80. https://doi.org/10.1080/15422119.2014.983128
Heffels P, Weber F, Schieber A (2015) Influence of accelerated solvent extraction and ultrasound-assisted extraction on the anthocyanin profile of different vaccinium species in the context of statistical models for authentication. J Agric Food Chem 63:7532–7538. https://doi.org/10.1021/acs.jafc.5b02255
Hidalgo GI, Almajano MP (2017) Red Fruits: Extraction of antioxidants, phenolic content, and radical scavenging determination: a review. Antioxidants 6:27. https://doi.org/10.3390/antiox6010007
Iglesias-Carres L, Mas-Capdevila A, Sancho-Pardo L, Bravo FI, Mulero M, Muguerza B, Arola-Arnal A (2018) Optimized extraction by response surface methodology used for the characterization and quantification of phenolic compounds in whole red grapes (Vitis vinifera). Nutrients 10:20. https://doi.org/10.3390/nu10121931
Klavins L, Kviesis J, Nakurte I, Klavins M (2018) Berry press residues as a valuable source of polyphenolics: extraction optimisation and analysis. LWT-Food Sci Technol 93:583–591. https://doi.org/10.1016/j.lwt.2018.04.021
Maatta-Riihinen KR, Kamal-Eldin A, Mattila PH, Gonzalez-Paramas AM, Torronen AR (2004) Distribution and contents of phenolic compounds in eighteen Scandinavian berry species. J Agric Food Chem 52:4477–4486. https://doi.org/10.1021/jf049595y
Moze S, Polak T, Gasperlin L, Koron D, Vanzo A, Ulrih NP, Abram V (2011) Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.). J Agric Food Chem 59:6998–7004. https://doi.org/10.1021/jf200765n
Myers RH, Montgomery DC (2002) Response surface methodology: process and product optimization using designed experiments WILEY SERIES IN PROBABILITY AND STATISTICS, 2nd edn. Wiley-Insterscience, Hoboken
Nour V, Trandafir I, Cosmulescu S (2015) Central composite design applied to optimize the hydroalcoholic extraction of bilberry (Vaccinium myrtillus L) fruits. J Food Biochem 39:179–188. https://doi.org/10.1111/jfbc.12119
Olas B (2018) Berry phenolic antioxidants—implications for human health? Front Pharmacol 9. https://doi.org/10.3389/fphar.2018.00078
Paassilta M, Moisio S, Jaakola L, Häggman H (2009) Voice of the Nordic Wild Berry Industry: a survey among the companies. Oulu University Press, Oulu
Pascu M, Pascu DE, Traistaru GA, Bunaciu AA, Orbeci C, Nechifor AC (2013) Kinetic studies of some biological active extracts with antioxidant properties. Rev Chim 64:785–790
Siesler HW, Ozaki Y, Kawata S, Heise HM (2007) Near infrared spectroscopy: principles, instruments, applications. Wiley-VCH, Weinheim
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299:152–178
Swadseh J (1996) HPLC practical and industrial applications. CRC Press, Boca Raton
Wrolstad RE, Durst RW, Lee J (2005) Tracking color and pigment changes in anthocyanin products. Trends Food Sci Technol 16:423–428. https://doi.org/10.1016/j.tifs.2005.03.019
Zoratti L, Palmieri L, Jaakola L, Haggman H (2015) Genetic diversity and population structure of an important wild berry crop. Aob Plants 7. https://doi.org/10.1093/aobpla/plv117
Acknowledgments
Part of the work was a student project carried out by Jimmie Klum and Simon Vikström.
Funding
Open access funding provided by Swedish University of Agricultural Sciences. This study was funded by Botnia-Atlantica 2014–2020, EU regional support (grant number 20200296).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Josefina Nyström declares that she has no conflict of interest. Ulla-Britt Östman declares that she has no conflict of interest. Torgny Mossing declares that he has no conflict of interest. Leif Hed declares that he has no conflict of interest. Paul Geladi declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nyström, J., Östman, UB., Mossing, T. et al. Solvent and Rotating Bed Reactor Extraction with One- and Two-Phase Solvents Applied to Bilberries (Vaccinium myrtillus) for Isolating Valuable Antioxidants. Food Anal. Methods 13, 933–941 (2020). https://doi.org/10.1007/s12161-020-01701-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-020-01701-x