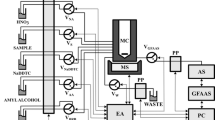
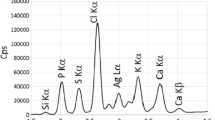
Abstract
In this work, a procedure is proposed for the direct analysis of powdered refreshments by fast sequential flame atomic absorption spectrometry (FS-F AAS), after a simple treatment of the sample. A full 23 factorial design was applied, and the variables chosen were as follows: acid concentration, acid type, and sonication time. Optimization provided the best conditions considering a mass of powdered refreshment of about 0.18 g diluted to a final volume of 15 mL with 0.5 mol L−1 HCl. The matrix effect was investigated, and external calibration was feasible for the determination of Cd, Co, Cu, Fe, Mg, Mn, Ni, Pb, and Zn. The limit of quantification (LoQ) obtained was between 0.6 (Cd) and 25 mg kg−1 (Pb). The analyte addition and recovery test were applied to evaluate the accuracy, and recovery percentage values ranging from 83.9 to 109.7% were obtained, which is considered adequate for quantitative analysis. Precision was expressed as relative standard deviation (% RSD); it was less than or equal to 5.0% (n = 7) for all analytes. The procedure was applied to 21 samples of powdered refreshment commercialized in Salvador (Brazil) and Buenos Aires (Argentina). The concentration range and average of the analytes in the samples (in mg kg−1) commercialized in Brazil (BZ) and Argentina (AG) were as follows: Co (BZ < 6.4–9.94, average 8.85; AG < 6.4–10.3, average 9.92), Fe (BZ < 6.9–376, average 270; AG < 6.9–32.81, average 29.11), Mg (BZ < 8.9–363, average 140; AG 770–3139, average 1464), Mn (BZ < 3.2–4.88, average 4.24; AG < 6.9), and Zn (BZ < 1.4–1.68, average 1.68; AG < 1.4). The concentrations of Cd, Cu, Ni, and Pb were lower than the LoQ of the proposed analytical method. For refreshment samples commercialized in Buenos Aires (Argentina), high concentrations of Mg were found in their chemical composition, but Mg had been used as anti-caking agent along with the other components.

Similar content being viewed by others
References
ANVISA (2004) Consulta Pública no. 80, de 13 de dezembro de 2004. D. O. U de 17/12/2004. Retrieved from http://www.anvisa.gov.br/divulga/consulta/index.htm%0A. Accessed 02 may 2018
ANVISA (2017) RDC N. 166, de 25 de Julho de 2017, Dispõe sobre a validação de métodos analíticos e dá outras providências. Ministerio Da Saúde, 2017, 1–21. Retrieved from http://portal.anvisa.gov.br/documents/10181/2721567/RDC_166_2017_COMP.pdf/d5fb92b3-6c6b-4130-8670-4e3263763401. Accessed 02 may 2018
Brandao GC, de Jesus RM, da Silva EGP, Ferreira SLC (2010) Use of slurry sampling for the direct determination of zinc in yogurt by high resolution-continuum source flame atomic absorption spectrometry. Talanta 81(4–5):1357–1359. https://doi.org/10.1016/j.talanta.2010.02.033
Da Costa SSL, Lima Pereira AC, Andrade Passos E, Hora Alves JDP, Borges Garcia CA, Oliveira Araujo RG (2013) Multivariate optimization of an analytical method for the analysis of dog and cat foods by ICP OES. Talanta 108:157–164. https://doi.org/10.1016/j.talanta.2013.03.002
Damodaram S, Parkin KL, Fennema OR (2010) Química dos alimentos de Fennema, 4th edn. Artmed, Porto Alegre
De Assis RA, Küchler IL, Miekeley N, Da Silveira CLP (2008) Elementos-traço e sódio em suco de uva: Aspectos nutricionais e toxicológicos. Quim Nova 31(8):1948–1952. https://doi.org/10.1590/S0100-40422008000800006
de Campos EMF, Rogoni TT, Massocatto CL, Diniz KM, Caetano J, Dragunski DC (2010) Quantificação de minerais em sucos industrializados. Arquivos de Ciências Da Saúde Da UNIPAR 14(1):11–16
Oliveira, C. H. De, Binotti, R. S., Quagliara, P. C., & Rebechi, M. (2006). Substâncias químicas presentes em sucos de frutas em pó comercializados no Brasil Chemical components present in powdered fruit juices commercially available in Brazil
de Sousa CP (2006) Segurança alimentar e doenças veiculadas por alimentos: utilização do grupo coliforme como um dos indicadores de qualidade de alimentos. Revista APS 9(1):83–88 Retrieved from http://www.ufjf.br/nates/files/2009/12/Seguranca.pdf
Ferrarezi AC, Dos Santos KO, Monteiro M (2010) Avaliação crítica da legislação brasileira de sucos de fruta, com ênfase no suco de fruta pronto para beber. Revista de Nutricao 23(4):667–677. https://doi.org/10.1590/S1415-52732010000400016
Ferreira SLC (2015) Introdução às técnicas de planejamento de experimentos. Vento Leste, Salvador
Ferreira SLC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandão GC, da Silva EGP, Portugal LA, dos Reis PS, Souza AS, dos Santos WNL (2007) Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta 597(2):179–186. https://doi.org/10.1016/j.aca.2007.07.011
Froes RES, Borges Neto W, Naveira RLP, Silva NC, Nascentes CC, da Silva JBB (2009) Exploratory analysis and inductively coupled plasma optical emission spectrometry (ICP OES) applied in the determination of metals in soft drinks. Microchem J 92(1):68–72. https://doi.org/10.1016/j.microc.2008.12.008
Galuch MB, Piccioli AFB, Neto ES, Fier N, Saldan NC, Garcia EE (2018) Microemulsion as sample preparation for direct flame atomic absorption spectrometry (FAAS) determination of total iron in crude and refined vegetable oils. J Braz Chem Soc 29(4):748–756. https://doi.org/10.21577/0103-5053.20170197
King T, Cole M, Farber JM, Eisenbrand G, Zabaras D, Fox EM, Hill JP (2017) Food safety for food security: relationship between global megatrends and developments in food safety. Trends Food Sci Technol 68:160–175. https://doi.org/10.1016/j.tifs.2017.08.014
Krug FJ, Rocha FRP (2016) Métodos de Preparo de Amostras: Fundamentos sobre métodos de preparo de amostras orgânicas e inorgânicas para análise elementar, 2nd edn. Edição do autor, Piracicaba
Miller JNM, Miller JC (2010) Statistics and chemometrics for analytical chemistry. In: Technometrics, 6th edn. Pearson, London. https://doi.org/10.1198/tech.2004.s248
Morgano MA, Queiroz SC d N, Ferreira MMC (1999) Determinação dos teores de minerais em sucos de frutas por espectrometria de emissão óptica em plasma indutivamente acoplado (ICP-OES). Cienc Tecnol Aliment 19(3). https://doi.org/10.1590/S0101-20611999000300009
Nascentes CC, Arruda MAZ, Nogueira ARA, Nóbrega JA (2004) Direct determination of Cu and Zn in fruit juices and bovine milk by thermospray flame furnace atomic absorption spectrometry. Talanta 64(4):912–917. https://doi.org/10.1016/j.talanta.2004.04.004
Oliveira E (2003) Sample preparation for atomic spectroscopy: evolution and future trends. J Braz Chem Soc 14(2):174–182. https://doi.org/10.1590/S0103-50532003000200004
Pinheiro AM, Fernandes AG, Fai AEC, Prado G, Do M, De Sousa PHM, Maia GA (2006) Avaliação química, físico-química e microbiológica de sucos de frutas integrais: abacaxi, caju e maracujá. Cienc Tecnol Aliment 26(1):98–103. https://doi.org/10.1590/S0101-20612006000100017
Pinho GP, Neves AA, Queiroz MELR, Silvério FO (2009) Efeito de matriz na quantificaçao de agrotóxico por cromatografia gasosa. Química Nova 32(4):987–995. https://doi.org/10.1590/S0100-40422009000400030
Projahn HD, Steeg U, Sanders J, Vanclay E (2004) Application of the reference-element technique for fast sequential flame atomic-absorption spectrometry. Anal Bioanal Chem 378(4):1083–1087. https://doi.org/10.1007/s00216-003-2200-y
Ribani M, Bottoli CBG, Collins CH, Jardim ICSF, Melo LFC (2004) Validação de métodos cromatográficos e eletroforéticos. Quim Nova 27(5):771–780. https://doi.org/10.1590/S0100-40422004000500017
Santos JS, Teixeira LSG, Araújo RGO, Fernandes AP, Korn MGA, Ferreira SLC (2011) Optimization of the operating conditions using factorial designs for determination of uranium by inductively coupled plasma optical emission spectrometry. Microchem J 97(2):113–117. https://doi.org/10.1016/j.microc.2010.08.002
Silva CS, Nóbrega JA (2002) Análise De Suspensões De Argilas Por Espectrometria De Emissão Óptica Com Plasma Induzido Com Configuração Axial. Quim Nova 25(6):1194–1196
Szymczycha-Madeja A, Welna M (2013) Evaluation of a simple and fast method for the multi-elemental analysis in commercial fruit juice samples using atomic emission spectrometry. Food Chem 141(4):3466–3472. https://doi.org/10.1016/j.foodchem.2013.06.067
Szymczycha-Madeja A, Welna M, Jedryczko D, Pohl P (2014) Developments and strategies in the spectrochemical elemental analysis of fruit juices. TrAC - Trends Anal Chem 55:68–80. https://doi.org/10.1016/j.trac.2013.12.005
Tormen L, Torres DP, Dittert IM, Araújo RGO, Frescura VLA, Curtius AJ (2011) Rapid assessment of metal contamination in commercial fruit juices by inductively coupled mass spectrometry after a simple dilution. J Food Compos Anal 24(1):95–102. https://doi.org/10.1016/j.jfca.2010.06.004
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB, Brazil), and Programa de Apoio a Jovens Professores Doutores (PROPESQ/UFBA, Brazil—Edital PROPCI/PROPG No. 004/2016), providing scholarship, financial support, and infrastructure.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Joelem de Carvalho Melo declares that he has no conflict of interest. Wellington Correia Carvalho declares that she has no conflict of interest. Elane S. Boa Morte declares that she has no conflict of interest. Rennan Geovanny Oliveira Araujo declares that he has no conflict of interest. Daniele Cristina M. B. Santos declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals.
Informed Consent
Not applicable for this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 29 kb)
Rights and permissions
About this article
Cite this article
Melo, J.C., Carvalho, W.C., Boa Morte, E.S. et al. Sequential Determination of Cd, Co, Cu, Fe, Mg, Mn, Ni, Pb, and Zn in Powdered Refreshments by FS-F AAS After a Simple Sample Treatment. Food Anal. Methods 13, 212–221 (2020). https://doi.org/10.1007/s12161-019-01589-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-019-01589-2