Abstract
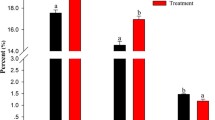
Here, we aimed to study the changes in proteome of tilapia during post-mortem storage. The effects of post-mortem storage time (0 and 7 days in ice storage) on the proteome of tilapia skeletal muscle was characterized by label-free proteomics. In total, 902 proteins were identified, of which 34 proteins underwent alterations that were statistically significant (P < 0.05). The 15 up-regulated and 19 down-regulated proteins were mainly structural proteins, followed by transcription and translation regulation, enzymes, and stress proteins. The top three enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways are metabolic pathways, tight junction, and ribosome. Two differentially abundant proteins, pgm1 and ldha, were selected for further verification using 0- and 7-day iced storage fish muscle. Western blotting analysis showed significantly lower levels of two proteins, ldha and pgm1, in ice-stored tilapia fillets than in fresh tilapia fillets. Our results indicate that meat tenderness is related to structural protein degradation and increased levels of lactic acid caused by enzymes and anaerobic conditions and provide an important basis toward enabling further understanding of the molecular mechanisms responsible for differences in fish texture and meat tenderness between fresh and ice-stored fish.





Similar content being viewed by others

References
Addis MF, Cappuccinelli R, Tedde V, Pagnozzi D, Porcu MC, Bonaglini E, Roggio T, Uzzau S (2010) Proteomic analysis of muscle tissue from gilthead sea bream (Sparus aurata L.) farmed in offshore floating cages. Aquaculture 309:245–252
Aebersold R, Mann M (2003) Mass spectrometry-based proteomics. Nature (UK Edition) 422:198–207
Altermann E, Klaenhammer TR (2005) PathwayVoyager: pathway mapping using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. BMC Genomics 6:60
Anderson MJ, Lonergan SM, Huff-Lonergan E (2014) Differences in phosphorylation of phosphoglucomutase 1 in beef steaks from the longissimus dorsi with high or low star probe values. Meat Sci 96:379–384
Aussanasuwannakul A, Kenney PB, Brannan RG, Slider SD, Salem M, Yao J (2010) Relating instrumental texture, determined by variable-blade and Allo-Kramer shear attachments, to sensory analysis of rainbow trout, Oncorhynchus mykiss, fillets. J Food Sci 75:365–374
Ayala MD, Abdel I, Santaella M, Martínez C, Periago MJ, Gil F et al (2010) Muscle tissue structural changes and texture development in sea bream, Sparus aurata L., during post-mortem storage. Food Sci Technol 43:465–475
Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR (2000) Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol 2:469–475
Bjarnadóttir SG, Hollung K, Høy M, Bendixen E, Codrea MC, Veiseth-Kent E (2012) Changes in protein abundance between tender and tough meat from bovine longissimus thoracis muscle assessed by isobaric Tag for Relative and Absolute Quantitation (iTRAQ) and 2-dimensional gel electrophoresis analysis. EN 90:2035–2043
Brunelle JK, Chandel NS (2002) Oxygen deprivation induced cell death: an update. Apoptosis 7:475–482
Carrera M, Losada V, Gallardo JM, Aubourg SP, Pieiro C (2008) Development of an indirect-α-actinin-based immunoassay for the evaluation of protein breakdown and quality loss in fish species subjected to different chilling methods. Int J Food Sci Technol 43:69–75
Chiozzi RZ, Capriotti AL, Cavaliere C, La Barbera G, Montone CM, Piovesana S, Laganà A (2018) Label-free shotgun proteomics approach to characterize muscle tissue from farmed and wild European sea bass (Dicentrarchus labrax). Food Anal Methods 11:292–3019
Ciaramella MA, Nair MN, Suman SP, Allen PJ, Schilling MW (2016) Differential abundance of muscle proteome in cultured channel catfish (Ictalurus punctatus) subjected to ante-mortem stressors and its impact on fillet quality. Comparative Biochemistry and Physiology - Part D: Genomics and Proteomics 20:10–18
D'Alessandro A, Zolla L (2013) Meat science: from proteomics to integrated omics towards system biology. J Proteome 78:558–577
D'Alessandro A, Marrocco C, Rinalducci S, Mirasole C, Failla S, Zolla L (2012) Chianina beef tenderness investigated through integrated omics. J Proteome 75:4381–4398
Dave D, Ghaly AE (2011) Meat spoilage mechanisms and preservation techniques: a critical review. Am J Agric Biol Sci 6:486–510
Durkin ME, Avner MR, Huh C, Yuan B, Thorgeirsson SS, Popescu NC (2005) DLC-1, a Rho GTPase-activating protein with tumor suppressor function, is essential for embryonic development. FEBS Lett 579:1191–1196
Gao X, Wu W, Ma C, Li X, Dai R (2016) Postmortem changes in sarcoplasmic proteins associated with color stability in lamb muscle analyzed by proteomics. Eur Food Res Technol 242:527–535
Godiksen H, Morzel M, Hyldig G, Jessen F (2009) Contribution of cathepsins B, L and D to muscle protein profiles correlated with texture in rainbow trout (Oncorhynchus mykiss). Food Chem 113:889–896
Golovan SP, Hakimov HA, Verschoor C, Schenkel F, Elsik C, Wright T et al (2009) Analysis of Sus scrofa liver proteome with isotope tagging for relative and absolute quantification (iTRAQ). Can J Anim Sci 89:164
International Commission on Microbiological Specifications for Foods (1986) Micro-organisms in foods: a publication of the International Commission on Microbiological Specifications for Foods (ICMSF) of the International Association of Microbiological Societies. University of Toronto Press, Toronto
Jedrzejas MJ (2000) Structure, function, and evolution of phosphoglycerate mutases: comparison with fructose-2,6-bisphosphatase, acid phosphatase, and alkaline phosphatase. Prog Biophys Mol Biol 73:263–287
Jensen LJ, Stark M, CS S, Creevey C, Muller J, Doerks T, Simonovic M, B P, von Mering C et al (2009) STRING 8—a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res 37:412–416
Jia X, Veiseth-Kent E, Grove H, Kuziora P, Aass L, Hildrum KI, Hollung K et al (2009) Peroxiredoxin-6-A potential protein marker for meat tenderness in bovine longissimus thoracis muscle. J Anim Sci 87:2391–2399
Johnston IA (1999) Muscle development and growth: potential implications for flesh quality in fish. Aquaculture 177:99–115
Jones BA, Haddock CW (1977) Bacterial respiration. Microbiol Mol Biol Rev 41:47–99
Kabbage M, Dickman MB (2008) The BAG proteins: a ubiquitous family of chaperone regulators. Cell Mol Life Sci 65:1390–1402
Kiran M, Naveena BM, Reddy KS, Shashikumar M, Reddy VR, Kulkarni VV, Rapole S, More TH (2015) Muscle-specific variation in buffalo (Bubalus bubalis) meat texture: biochemical, ultrastructural and proteome characterization. J Texture Stud 46:254–261
Kjærsgård IVH, Jessen F (2004) Two-dimensional gel electrophoresis detection of protein oxidation in fresh and tainted rainbow trout muscle. Food Chem 52:7101 − 7107
Kjrsgrd IVH, Jessen F (2003) Proteome analysis elucidating post-mortem changes in cod (Gadus morhua) muscle proteins. J Agric Food Chem 51:3985–3991
Laville E, Sayd T, Morzel M, Blinet S, Chambon C, Lepetit J, Renand G, Hocquette JF̧ (2009) Proteome changes during meat aging in tough and tender beef suggest the importance of apoptosis and protein solubility for beef aging and tenderization. J Agric Food Chem 57:10755–10764
Li T, Li J, Hu W, Chen J, Li H (2014) Protein changes in post mortem large yellow croaker (Pseudosciaena crocea) monitored by SDS-PAGE and proteome analysis. Food Control 41:49–55
Lim SA, Ahmed MU (2016) A label free electrochemical immunosensor for sensitive detection of porcine serum albumin as a marker for pork adulteration in raw meat. Food Chem 206:197–203
Lomiwes D, Farouk MM, Frost DA, Dobbie PM, Young OA (2013) Small heat shock proteins and toughness in intermediate pHu beef. Meat Sci 95:472–479
Marino R, Albenzio M, Della Malva A, Santillo A, Loizzo P, Sevi A (2013) Proteolytic pattern of myofibrillar protein and meat tenderness as affected by breed and aging time. Meat Sci 95:281–287
Mohanty BP, Mitra T, Banerjee S, Bhattacharjee S, Mahanty A, Ganguly S, Purohit GK, Karunakaran D, Mohanty S (2015) Proteomic profiling of white muscle from freshwater catfish Rita rita. Fish Physiol Biochem 41:789–802
Oliván M, Fernández-Suárez V, Díaz-Martínez F, Sierra V, Coto-Montes A, de Luxán-Delgado B et al (2016) Identification of biomarkers of stress in meat of pigs managed under different mixing treatments. Br Biotechnol J 11:1–13
Ortiz C, Cardemil L (2001) Heat-shock responses in two leguminous plants: a comparative study. J Exp Bot 52:1711–1719
Perillo NL, Pace KE, Seilhamer JJ, Baum LG (1995) Apoptosis of T cells mediated by galectin-1. Nature 378:736–739
Picard B, Lefèvre F, Lebret B (2012) Meat and fish flesh quality improvement with proteomic applications. Animal Frontiers 2:18–25
Piovesana S, Capriotti AL, Caruso G, Cavaliere C, La Barbera G, Chiozzi RZ et al (2015) Labeling and label free shotgun proteomics approaches to characterize muscle tissue from farmed and wild gilthead sea bream (Sparus aurata). J Chromatogr A 1428:193–201
Salem M, Kenney PB, Rexroad CE, Yao J (2010) Proteomic signature of muscle atrophy in rainbow trout. J Proteome 73:778–789
Sarah SA, Faradalila WN, Salwani MS, Amin I, Karsani SA, Sazili AQ (2016) LC–QTOF-MS identification of porcine-specific peptide in heat treated pork identifies candidate markers for meat species determination. Food Chem 199:157–164
Silva JA, Patarata L, Martins C (1999) Influence of ultimate pH on bovine meat tenderness during ageing. Meat Sci 52:453–459
Subbaiah K, Majumdar RK, Choudhury J, Priyadarshini BM, Dhar B, Roy D, Saha A, Maurya P (2015) Protein degradation and instrumental textural changes in fresh Nile tilapia (Oreochromis niloticus) during frozen storage. Journal of Food Processing and Preservation 39:2206–2214
Suvanich V, Jahncke ML, Marshall DL (2000) Changes in selected chemical quality characteristics of channel catfish frame mince during chill and frozen storage. J Food Sci 65:24–29
Vizcaíno JA, Csordas A, Del-Toro N, Dianes JA, Griss J, Lavidas I, Mayer G, Perez-Riverol Y, Reisinger F, Ternent T, Xu Q, Wang R, Hermjakob H (2016) update of the PRIDE database and its related tools. Nucleic Acids Res 44:447–456
Wang Z, He F, Rao W, Ni N, Shen Qand Zhang D (2016) Proteomic analysis of goat Longissimus dorsi muscles with different drip loss values related to meat quality traits. Food Sci Biotechnol 25:425–431
Wen W, Nie Y, Luo X, Guan J, Liu H, Lu X, Sun Q (2015) Proteomic analysis of yak Longissimus dorsi muscle by two-dimensional electrophoresis. Meat Sci 101:160–161
Wu W, Yu Q, Fu Y, Tian X, Jia F, Li X et al (2016) Towards muscle-specific meat color stability of Chinese Luxi yellow cattle: a proteomic insight into post-mortem storage. J Proteome 147:108–118
Yu QQ, Wu W, Tian XJ, Hou M, Dai RT, Li XM (2017) Unraveling proteome changes of Holstein beef M. semitendinosus and its relationship to meat discoloration during post-mortem storage analyzed by label-free mass spectrometry. J Proteome 154:85–93
Yuan M, Zhou R, She B, Xu H, Wang J, Wei L (2014) Expression and clinical significance of STIP1 in papillary thyroid carcinoma. Tumor Biol 35:2391–2395
Zhang J, Gao Y, Lu Q, Ss R, Zhang H (2015) iTRAQ-based quantitative proteomic analysis of longissimus muscle from growing pigs with dietary supplementation of non-starch polysaccharide enzymes. J Zhejiang Univ-Sci B(Biomedicine & Biotechnology) 465-478:16
Zhao Z, Wu F, Ding S, Sun L, Liu Z, Ding K, Lu J (2015) Label-free quantitative proteomic analysis reveals potential biomarkers and pathways in renal cell carcinoma. Tumor Biol 36:939–951
Zhu Y, Zhang K, Ma L, Huo N, Yang H, Hao J (2015) Sensory, physicochemical, and microbiological changes in vacuum packed channel catfish (Clarias lazera) patties during controlled freezing-point storage. Food Sci Biotechnol 24:1249–1256
Funding
This research project was funded by the Natural Science Foundation of China [grant numbers 31601533 and 31401563]; National Key Technology Support Program [grant number 2015BAD17B03-2]; National Modern Agriculture (tilapia) Industrial Technology System Specific [grant number CARS-49]; Science and Technology Planning Project of Guangdong Province(grant number 2016A020210025); Pearl River S&T Nova Program of Guangzhou (grant number 201710010167); Central Institutes of Public Welfare Projects (SCSFRI, CAFS)(grant number 2015B06YQ01); and National agricultural product quality safety risk assessment project plan [grant number GJFP201601504].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Yanfu He declares that she has no conflict of interest. Hui Huang declares that she has no conflict of interest. Laihao Li declares that he has no conflict of interest. Xianqing Yang declares that he has no conflict of interest.
Ethical Approval
The studies with animals were performed following all institutional and national guidelines for the care and use of laboratory animals.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
He, Y., Huang, H., Li, LH. et al. Label-Free Proteomics of Tilapia Fillets and Their Relationship with Meat Texture During Post-Mortem Storage. Food Anal. Methods 11, 3023–3033 (2018). https://doi.org/10.1007/s12161-018-1273-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-018-1273-3