Abstract
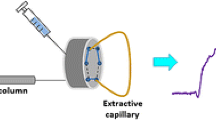
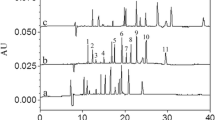
An integrative coupling approach of headspace in-tube microextraction (HS-ITME) and reverse-flow micellar electrokinetic capillary chromatography (RF-MECC) was proposed for determination of phthalic acid esters (PAEs). In the novel method, by placing a capillary filled with background electrolyte (BGE) of RF-MECC in the HS above the sample solution, the PAEs were extracted into the acceptor phase in the capillary. Then, the extracts were separated and determined by RF-MECC. The influence of some essential BGE components such as sodium dodecyl sulfate (SDS) and organic modifier concentrations were investigated. Extraction parameters were also systematically investigated, including the extraction temperature, extraction time, salt concentration, and volume of the sample solution. Under the optimized conditions, enrichment factors for three PAEs were 169, 2534, and 1526, respectively. The proposed method provided a good linearity, low limits of detection, and good repeatability (RSDs below 5.44%, n = 5). This method was then utilized to analyze the three PAEs in tap water, seawater, and beverage samples. The results indicated that the developed method is an excellent alternative for the PAE routine analysis in the food field.





Similar content being viewed by others
References
Al-Natsheh M, Alawi M, Fayyad M (2015) Simultaneous GCMS determination of eight phthalates in total and migrated portions of plasticized polymeric toys and childcare articles. J Chromatogr B985:103–109
Banitaba MH, Davarani SSH, Pourahadi A (2013) Solid-phase microextraction of phthalate esters from aqueous media by electrophoretically deposited TiO2 nanoparticles on a stainless steel fiber. J Chromatogr A 1283:1–8
Cinelli G, Avino P, Notardonato I (2013) Rapid analysis of six phthalate esters in wine by ultrasound-vortex-assisted dispersive liquid–liquid micro-extraction coupled with gas chromatography-flame ionization detector or gas chromatography–ion trap mass spectrometry. Anal Chim Acta 769:72–78
Farajzadeh MA, Mogaddam MRA (2012) Air-assisted liquid–liquid microextraction method as a novel microextraction technique; application in extraction and preconcentration of phthalate esters in aqueous sample followed by gas chromatography–flame ionization detection. Anal Chim Acta 728:31–38
Fierens T, Holderbeke MV, Willems H (2013) Transfer of eight phthalates through the milk chain—a case study. Environ Int 51:1–7
Gómara B, Lebrón-Aguilar R, González MJ (2015) Insight into the retention processes of phthalate metabolites on different liquid chromatography stationary phases for the development of improved separation methods. J Chromatogr A 1423:86–95
Hayasaka Y (2014) Analysis of phthalates in wine using liquid chromatography tandem mass spectrometry combined with a hold-back column: chromatographic strategy to avoid the influence of pre-existing phthalate contamination in a liquid chromatography system. J Chromatogr A 1372:120–127
Heudorf U, Mersch-Sundermann V, Angerer J (2007) Phthalates: toxicology and exposure. Int J Hyg Environ Health 210:623–634
Kim SA, Kim SH, Kim IS (2013) Simultaneous determination of bioactive phenolic compounds in the stem extract of Rhus verniciflua, stokes by high performance liquid chromatography. Food Chem 141:3813–3819
Li HY, Shi YT, Zeng QF (2006) Simultaneous determination of phthalate esters by micelle capillary electrophoresis. China Water & Wastewater 22:88–90
Li J, Su Q, Li KY, Sun CF, Zhang WB (2013) Rapid analysis of phthalates in beverage and alcoholic samples by multi-walled carbon nanotubes/silica reinforced hollow fibre-solid phase microextraction. Food Chem 141:3714–3720
Li X, Wang J, Zhang Q (2015) Advances on the development of detection methods for the phthalate esters in food. Chin J Chromatogr 11:1147–1154
Ling DS, Xie HY, He YZ (2010) Determination of preservatives by integrative coupling method of headspace liquid-phase microextraction and capillary zone electrophoresis. J Chromatogr A 1217:7807–7811
Liu X, Sun Z, Chen G (2015) Determination of phthalate esters in environmental water by magnetic Zeolitic Imidazolate Framework-8 solid-phase extraction coupled with high-performance liquid chromatography. J Chromatogr A 1409:46–52
Miller JL, Khaledi MG, Shea D (1997) Separation of polycyclic aromatic hydrocarbons by nonaqueous capillary electrophoresis using charge-transfer complexation with planar organic cations. Anal Chem 69:1223–1229
Mtibe A, Msagati TAM, Mishra AK (2012) Determination of phthalate ester plasticizers in the aquatic environment using hollow fibre supported liquid membranes. Phys Chem Earth 50-52:239–242
Okada T (1997) Non-aqueous capillary electrophoretic separation of Brønsted acids as heteroconjugated anions. J Chromatogr A 771:275–284
Ranjbari E, Hadjmohammadi MR (2012) Magnetic stirring-assisted dispersive liquid–liquid microextraction followed by high performance liquid chromatography for determination of phthalate esters in drinking and environmental water samples. Talanta 100:447–453
Stales CA, Peterson DR, Parkerton TF (1997) The environmental fate of phthalate esters: a literature review. Chemosphere 35:667–749
Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternard CL, Sulivan S, Teague JL (2005) Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Persp 113(8):1056–1061
Terabe S, Otsuka K, Ando T (1985) Electrokinetic chromatography with micellar solution and open-tubular capillary. Anal Chem 57:834–841
Terabe S, Katsura T, Okada Y (1993) Measurement of thermodynamic quantities of micellar solubilization by micellar electrokinetic chromatography with sodium dodecyl sulfate. J Microcolumn Sep 5:23–33
Viñas P, Campillo N, Pastor-Belda M (2015) Determination of phthalate esters in cleaning and personal careproducts by dispersive liquid–liquid microextraction and liquid chromatography–tandem mass spectrometry. J Chromatogr A 1376:18–25
Walbroehl Y, Jorgenson JW (1984) On-column UV absorption detector for open tubular capillary zone electrophoresis. J Chromatogr A 315:135–143
Wams TJ (1987) Diethylhexylphthalate as an environmental contaminant: a review. Sci Total Environ 66:1–16
Wang LL, Huang WH, Shao XM (2003) Development of the analysis of environmental endocrine disruptor compounds. J Ana Sci 19:179–184
Xu J, Liang P, Zhang TZ (2007) Dynamic liquid-phase microextraction of three phthalate esters from water samples and determination by gas chromatography. Anal Chim Acta 597:1–5
Xu G, Li F, Wang Q (2008) Occurrence and degradation characteristics of dibutyl phthalate (DBP) and di-(2-ethylhexyl) phthalate (DEHP) in typical agricultural soils of China. Scl Total Environ 393:333–340
Xu D, Deng X, Fang E (2014) Determination of 23 phthalic acid esters in food by liquid chromatography tandem mass spectrometry. J Chromatogr A 1324:49–56
Ye Q, Liu L, Chen Z (2014) Analysis of phthalate acid esters in environmental water by magnetic graphene solid phase extraction coupled with gas chromatography–mass spectrometry. J Chromatogr A 1329:24–29
Yue M-E, Xu J, Hou WG (2015) Determination of five phthalate esters in running water and milk by micellar electrokinetic capillary chromatography. J Anal Chem 70:1147–1152
Yue M-E, Li Q, Xu J, Jiang T-F (2016) Headspace in-tube microextraction coupled with capillary electrophoresis for detection of bromophenols in water and Trachypenaeus curvirostris. Food Anal Method 9:1912–1918
Zhang H, Chen X, Jiang X (2011) Determination of phthalate esters in water samples by ionic liquid cold-induced aggregation dispersive liquid–liquid microextraction coupled with high-performance liquid chromatography. Anal Chim Acta 689:137–142
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Science and Technology Development Plan Project of Shandong Province (2014GSF121003) and the Natural Science Foundation of Shandong Province (ZR2015BL019).
Conflict of Interest
Mei-E Yue declares that she has no conflict of interest. Qian Li declares that she has no conflict of interest. Ting-Fu Jiang declares that she has no conflict of interest. Jie Xu declares that she has no conflict of interest.
Informed Consent
Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Ethical Standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Yue, ME., Lin, Q., Li, Q. et al. Determination of PAEs by Integrative Coupling Method of Headspace in-Tube Microextraction and Reverse-Flow Micellar Electrokinetic Capillary Chromatography. Food Anal. Methods 10, 3565–3571 (2017). https://doi.org/10.1007/s12161-017-0917-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0917-z