Abstract
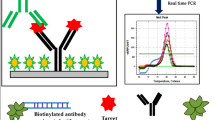
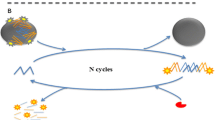
Staphylococcus aureus is a major foodborne pathogen worldwide, and as little as 1 μg of staphylococcal enterotoxins (SEs) can cause food poisoning. Among SEs, enterotoxin A is the most common toxin that causes staphylococcal food poisoning. Hence, this work has developed a dual-labeled PCR-based immunofluorescent assay using anti-digoxigenin (DIG) antibody-tagged immunomagnetic beads (IMBs) as the capture reagent and cocktail-sized NeutrAvidin-tagged liposomal nanovesicles (NA-LNs) that encapsulate fluorescent dyes as the detection reagent. In this approach, the amplicon of sea gene was doubly labeled with DIG and biotin using modified primers and biotin-11-dUTP. The system depends on the immunocapture of IMB to pre-concentrate the labeled amplicons, which were further quantified using cocktail-sized NA-LNs, based on the release and subsequent measurement of encapsulated fluorescent dyes following the lysis of NA-LNs. After optimization, the developed assay could detect S. aureus and differentiate it from other common foodborne bacteria, such as Salmonella enterica and Escherichia coli, with a limit of detection (LOD) of 101 CFU mL−1 without pre-enrichment. With a 2-h pre-enrichment, this developed assay could detect as little as 1 CFU in 25 mL of milk within a workday. Hence, this work established a rapid and sensitive PCR-based immunofluorescent assay using liposomal nanovesicles as an instant signal enhancer to detect the contamination of enterotoxic S. aureus in milk.






Similar content being viewed by others
References
Argudín MÁ, Mendoza MC, Rodicio MR (2010) Food poisoning and Staphylococcus aureus enterotoxins. Toxins 2(7):1751–1773
Asao T, Kumeda Y, Kawai T et al (2003) An extensive outbreak of staphylococcal food poisoning due to low-fat milk in Japan: estimation of enterotoxin A in the incriminated milk and powdered skim milk. Epidemiol Infect 130(1):33–40
Aycicek H, Cakiroglu S, Stevenson TH (2005) Incidence of Staphylococcus aureus in ready-to-eat meals from military cafeterias in Ankara, Turkey. Food Control 16(6):531–534
Babu D, Muriana PM (2011) Immunomagnetic bead-based recovery and real time quantitative PCR (RT iq-PCR) for sensitive quantification of aflatoxin B (1). J Microbiol Methods 86(2):188–194
Baird RM, Lee WH (1995) Media used in the detection and enumeration of Staphylococcus aureus. Int J Food Microbiol 26(1):15–24
Balaban N, Rasooly A (2000) Staphylococcal enterotoxins. J Food Microbiol 61(1):1–10
Bartlett GR (1959) Phosphorus assay in column chromatography. J Biol Chem 234(3):466–468
Bayer EA, Ben-Hur H, Wilchek M (1990) Isolation and properties of streptavidin. Methods Enzymol 184:80–89
Borsa BA, Tuna BG, Hernandez FJ et al (2016) Staphylococcus aureus detection in blood samples by silica nanoparticle-oligonucleotides conjugates. Biosens Bioelectron 15(86):27–32
Cha JO, Lee JK, Jung YH et al (2006) Molecular analysis of Staphylococcus aureus isolates associated with staphylococcal food poisoning in South Korea. J Appl Microbiol 101(4):864–871
Chu PT, Wen HW (2013) Sensitive detection and quantification of gliadin contamination in gluten-free food with immunomagnetic beads based liposomal fluorescence immunoassay. Anal Chim Acta 787:246–253
Chu PT, Hsieh MF, Yin SY, Wen HW (2009) Development of a rapid and sensitive immunomagnetic-bead based assay for detection of Bacillus cereus in milk. Eur Food Res Technol 229(1):73–81
Conlan JV, Khounsy S, Blacksell SD et al (2009) Development and evaluation of a rapid immunomagnetic bead assay for the detection of classical swine fever virus antigen. Trop Anim Health Prod 41(6):913–920
Edwards KA, Baeumner AJ (2006a) Optimization of DNA-tagged liposomes for use in microtiter plate analyses. Anal Bioanal Chem 386(6):1613–1623
Edwards KA, Baeumner AJ (2006b) Optimization of DNA-tagged dye-encapsulating liposomes for lateral-flow assays based on sandwich hybridization. Anal Bioanal Chem 386(5):1335–1343
European Food Safety Authority (EFSA), European Centre for Disease Prevention and Control (ECDC) (2015) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J 13(3991):1–165
GB 4789.10 (2010) National food safety standard Food microbiological examination: Staphylococcus aureus. National Standard of the People’s Republic of China. http://china.nlambassade.org/binaries/content/assets/postenweb/c/china/zaken-doen-in-china/2013/productstandaarden-zuivel/gb4789.10-2010-food-microbiological-examination---staphylococcus-aureus.pdf. Accessed 27 Feb 2017
Gomes LI, Dos Santos Marques LH, Enk MJ et al (2010) Development and evaluation of a sensitive PCR-ELISA system for detection of schistosoma infection in feces. PLoS Negl Trop Dis 4(4):e664
Hiller Y, Gershoni JM, Bayer EA, Wilchek M (1987) Biotin binding to avidin. Oligosaccharide side chain not required for ligand association. Biochem J 248(1):167–171
Jay JM, Loessner MJ, Golden DA (2005) Staphylococcal gastroenteritis. In: Heldman DR (ed) Modern Food Microbiology, 7th edn. Springer, New York, pp 545–566
Jin W, Yamada K, Ikami M et al (2013) Application of IgY to sandwich enzyme-linked immunosorbent assays, lateral flow devices, and immunopillar chips for detecting staphylococcal enterotoxins in milk and dairy products. J Microbiol Methods 92(3):323–331
Jung Y, Jeong JY, Chung BH (2008a) Recent advances in immobilization methods of antibodies on solid supports. Analyst 133(6):697–701
Jung YK, Kim TW, Jung C et al (2008b) A polydiacetylene microchip based on a biotin-streptavidin interaction for the diagnosis of pathogen infections. Small 4(10):1778–1784
Kérouanton A, Hennekinne JA, Letertre C et al (2007) Characterization of Staphylococcus aureus strains associated with food poisoning outbreaks in France. Int J Food Microbiol 115(3):369–375
Korpela J (1984) Avidin, a high affinity biotin-binding protein, as a tool and subject of biological research. Med Biol 62(1):5–26
Leserman LD, Barbet J, Kourilsky F, Weinstein JN (1980) Targeting to cells of fluorescent liposomes covalently coupled with monoclonal antibody or protein A. Nature 288:602–604
Lü JH, Li HK, An HJ et al (2004) Positioning isolation and biochemical analysis of single DNA molecules based on nanomanipulation and single-molecule PCR. J Am Chem Soc 126(36):11136–11137
Mazurek J, Salehi E, Propes D et al (2004) A multistate outbreak of Salmonella enterica serotype typhimurium infection linked to raw milk consumption—Ohio, 2003. J Food Prot 67(10):2165–2170
Mehrotra M, Wang G, Johnson WM (2000) Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J Clin Microbiol 38(3):1032–1035
Oliver SP, Jayarao BM, Almeida RA (2005) Foodborne pathogens in milk and the dairy farm environment: food safety and public health implications. Foodborne Pathog Dis 2(2):115–129
Paul N, Yee J (2010) PCR incorporation of modified dNTPs: the substrate properties of biotinylated dNTPs. BioTechniques 48:333–334
Shin J, Kim M (2008) Development of liposome immunoassay for Salmonella spp. using immunomagnetic separation and immunoliposome. J Microbiol Biotechnol 18(10):1689–1694
Sung YJ, Suk HJ, Sung HY et al (2013) Novel antibody/gold nanoparticle/magnetic nanoparticle nanocomposites for immunomagnetic separation and rapid colorimetric detection of Staphylococcus aureus in milk. Biosens Bioelectron 15(43):432–439
Tamanaha CR, Mulvaney SP, Rife JC, Whitman LJ (2008) Magnetic labeling, detection, and system integration. Biosens Bioelectron 24(1):1–13
Tasara T, Angerer B, Damond M et al (2003) Incorporation of reporter molecule-labeled nucleotides by DNA polymerases. II. High-density labeling of natural DNA. Nucleic Acids Res 31(10):2636–2646
Vaidya HC, Wolf BA, Garrett N et al (1988) Extremely high values of prostate-specific antigen in patients with adenocarcinoma of the prostate; demonstration of the “hook effect”. Clin Chem 34:2175–2177
van Roy N, Mangelschots K, Speleman F (1993) Improved immunocytochemical detection of biotinylated probes with Neutralite avidin. Trends Genet 9(3):71–72
Wellman AD, Sepaniak MJ (2006) Magnetically-assisted transport evanescent field fluoroimmunoassay. Anal Chem 78(13):4450–4456
Wen HW, Decory TR, Borejsza-Wysocki W, Durst RA (2006) Investigation of NeutrAvidin-tagged liposomal nanovesicles as universal detection reagents for bioanalytical assays. Talanta 68(4):1264–1272
Wen HW, Tsai WC, Chu PT, Yin HY (2016) Applications of liposomal nanovesicles in lateral flow assays. In: Edwards KA (ed) Liposomes in analytical methodologies. Pan Stanford, Singapore, pp 83–138
Wieneke AA, Roberts D, Gilbert RJ (1993) Staphylococcal food poisoning in the United Kingdom, 1969-90. Epidemiol Infect 110(3):519–531
Xu M, Wang R, Li Y (2016) Rapid detection of Escherichia coli O157:H7 and Salmonella Typhimurium in foods using an electrochemical immunosensor based on screen-printed interdigitated microelectrode and immunomagnetic separation. Talanta 148:200–208
Yin HY, Fang TJ, Wen HW (2016) Combined multiplex loop-mediated isothermal amplification with lateral flow assay to detect sea and seb genes of enterotoxic Staphylococcus aureus. Lett Appl Microbiol 63(1):16–24
Yu J, Zhang Y, Zhang Y et al (2016) Sensitive and rapid detection of Staphylococcus aureus in milk via cell binding domain of lysine. Biosens Bioelectron 15(77):366–371
Zacco E, Pividori MI, Alegret S et al (2006) Electrochemical magneto immunosensing strategy for the detection of pesticides residues. Anal Chem 78(6):1780–1788
Zaytseva NV, Montagna RA, Baeumner AJ (2005) Microfluidic biosensor for the serotype-specific detection of dengue virus RNA. Anal Chem 77(23):7520–7527
Acknowledgements
The authors would like to thank Ted Knoy for his editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by National Science Council of the Republic of China, Taiwan (No. NSC 99-2627-M-005-002).
Ethical Approval
This article does not contain any studies with human or animal subjects.
Conflict of Interest
Hsin-Yi Yin declares that he has no conflict of interest. Hsiao-Wei Wen declares that he has no conflict of interest.
Informed Consent
Not applicable.
Electronic Supplementary Material
ESM 1
Optimization of amount of antibody coated on protein G magnetic beads. Different concentrations of anti-digoxigenin (DIG) antibodies (0–250 μg mL-1) were mixed with protein G magnetic beads for 30 min at 37 oC to produce immunomagnetic beads (IMBs), which were used in capturing DIG and biotin dual-labeled amplicons. Bound amplicons were measured using streptavidin conjugated horseradish peroxidase (SA-HRP). (DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Yin, HY., Wen, HW. Dual-Labeled PCR-Based Immunofluorescent Assay for the Rapid and Sensitive Detection of Enterotoxic Staphylococcus aureus Using Cocktail-Sized Liposomal Nanovesicles as Signal Enhancer. Food Anal. Methods 10, 3264–3274 (2017). https://doi.org/10.1007/s12161-017-0893-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0893-3