Abstract
Polyether antibiotics have been widely used for the treatment and prevention of coccidiosis in chicken farming. In the present study, an efficient, simple and inexpensive competitive indirect enzyme-linked immunosorbent assay (ciELISA) method based on immunomagnetic sample clean up was developed for salinomycin. Monoclonal antibodies were immobilized on the surface of carboxylic acid magnetic beads (2.8 μm in diameter). After a simple extraction, residues in sample extracts were specifically adsorbed and the supernatant removed by magnetic separation. Analytes retained on the beads were then released by elution prior to ciELISA. The limit of detection for salinomycin in chicken muscle and liver was 22 and 18 ng mL−1, respectively, and the linear quantitative range was 47–653 ng mL−1. Intra-assay recoveries ranged from 86.00 to 99.32%, and inter-assay recoveries were between 85.68 and 96.34%. The inhibition efficiency and sensitivity of this method was improved compared with traditional hydrophilic-lipophilic balance column clean up ciELISA. Furthermore, the convenience and repeatability of the immunomagnetic cleanup renders the new method of high value for the analysis of drug residues in complex matrices.

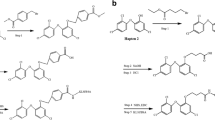
The test method of schematic diagram




Similar content being viewed by others
References
Aguilar-Caballos MP, Gómez-Hens A, Perez-Bendito D (1999) Determination of lasalocid with sensitized terbium (III) luminescence detection. Talanta 48(1):209–217
Babu D, Muriana PM (2011) Immunomagnetic bead-based recovery and real time quantitative PCR (RT iq-PCR) for sensitive quantification of aflatoxin B1. Microbiol Meth 86(2):188–194
Caragata M, Shah AK, Schulz BL, Hill MM, Punyadeera C (2016) Enrichment and identification of glycoproteins in human saliva using lectin magnetic bead arrays. Anal Biochem 497:76–82
Castilho MDS, Laube T, Yamanaka H, Alegret S, Pividori MI (2011) Magneto immunoassays for plasmodium falciparum histidine-rich protein 2 related to malaria based on magnetic nanoparticles. Anal Chem 83(14):5570–5577
Chen Y, Qi C, Xie M, Wang D, Wang Y, Xue Q, Li J, Chen Y (2013) Immunosensor based on magnetic relaxation switch and biotin–streptavidin system for the detection of kanamycin in milk. Biosens Bioelectron 39(1):112–117
Decory TR, Durst RA, Zimmerman SJ, Garringer LA, Paluca G, Decory HH, Montagna RA (2005) Development of an immunomagnetic bead-Immunoliposome fluorescence assay for rapid detection of Escherichia coli O157:H7 in aqueous samples and comparison of the assay with a standard microbiological method. Appl Environ Microb 71(4):1856–1864
Dowling L (1992) Ionophore toxicity in chickens: a review of pathology and diagnosis. Avian Pathol 21(3):355–368
Electronic Code of Federal Regulations (2016) Food and Drug Administration, the United States
Gijs MAM (2004) Magnetic bead handling on-chip: new opportunities for analytical applications. Microfluid Nanofluid 1(1):22–40
Godfrey MAJ, Luckey MF, Kwasowski P (1997) IAC/cELISA detection of monensin elimination from chicken tissues, following oral therapeutic dosing. Food Addit Contam 14(3):281–286
Hock B, Dankwardt A, Kramer K, Marx A (1995) Immunochemical techniques: antibody production for pesticide analysis. A review. Anal Chim Acta 311(3):393–405
Jerez A, Chihuailaf RH, Gai MN, Noro M, Wittwer F (2013) Development and validation of an HPLC method to determine lasalocid in raw milk samples from dairy cows. Revista Científica 23(6):537–542
List of maximum residue limits (MRLs) for veterinary drugs in foods (2015) Health Canada. http://www.hc-sc.gc.ca/dhp-mps/vet/mrl-lmr/mrl-lmr_versus_new-nouveau-eng.php
Liu F, Pawan KC, Zhang G, Zhe J (2016) Microfluidic magnetic bead assay for cell detection. Anal Chem 88(1):711–717
Matabudul DK, Lumley ID, Points JS (2002) The determination of 5 anticoccidial drugs (nicarbazin, lasalocid, monensin, salinomycin and narasin) in animal livers and eggs by liquid chromatography linked with tandem mass spectrometry (LC-MS-MS). Analyst 127(6):760–768
Moran JW, Turner JM, Coleman MR (1995) Determination of monensin in edible bovine-tissues and milk by liquid-chromatography. J AOAC Int 78(3):668–673
Muldoon MT, Elissalde MH, Beier RC, Stanker LH (1995) Development and validation of a monoclonal antibody-based enzyme-linked immunosorbent assay for salinomycin in chicken liver tissue. J Agr Food Chem 43(6):1745–1750
Nász S, Debreczeni L, Rikker T, Eke Z (2012) Development and validation of a liquid chromatographic-tandem mass spectrometric method for determination of eleven coccidiostats in milk. Food Chem 133(2):536–543
Olejnik M, Szprengier-Juszkiewicz T, Jedziniak P (2009) Multi-residue confirmatory method for the determination of twelve coccidiostats in chicken liver using liquid chromatography tandem mass spectrometry. J Chromatogr A 1216(46):8141–8148
Olejnik M, Szprengier-Juszkiewicz T, Jedziniak P (2010) Confirmatory method for determination of coccidiostats in eggs. B Vet I Pulawy 54(3):327–333
Positive list system for agricultural chemical residues—provisional MRLs list (2006) Ministry of Health, Labour and Welfare, Japan
Ra Y-K, Li C, Jiang H-Y, Zhang S-X, Zhao S-J, Li X-W, Shen J-Z (2008) Immunoaffinity chromatography clean-up and LC for analysis of salinomycin and narasin in chicken muscle. Chromatographia 68(9–10):701–706
Richardson J, Hawkins P, Luxton R (2001) The use of coated paramagnetic particles as a physical label in a magneto-immunoassay. Biosens Bioelectron 16(9–12):989–993
Rosén J (2001) Efficient and sensitive screening and confirmation of residues of selected polyether ionophore antibiotics in liver and eggs by liquid chromatography-electrospray tandem mass spectrometry. Analyst 126(11):1990–1995
Stanker LH, Elissalde MH, Rowe LD, Beier RC, Nasr MIAEA (1994) Detection of coccidiostats by immunoassay. Food Agr Immunol 6(1):45–54
Tang D, Yu Y, Niessner R, Miro M, Knopp D (2010) Magnetic bead-based fluorescence immunoassay for aflatoxin B1 in food using biofunctionalized rhodamine B-doped silica nanoparticles. Analyst 135(10):2661–2667
The European Union (2010) Commission regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin
Uddayasankar U, Zhang Z, Shergill RT, Gradinaru CC, Krull UJ (2014) Isolation of monovalent quantum dot - nucleic acid conjugates using magnetic beads. Bioconjug Chem 25(7):1342–1350
Ward TL, Moran JW, Turner JM, Coleman MR (2005) Validation of a method for the determination of narasin in the edible tissues of chickens by liquid chromatography. J AOAC Int 88(1):95–101
Watanabe H, Satake A, Kido Y, Tsuji A (2001) Monoclonal-based enzyme-linked immunosorbent assay and immunochromatographic rapid assay for salinomycin. Anal Chim Acta 437(1):31–38
Zacco E, Adrián J, Galve R, Marco MP, Alegret S, Pividori MI (2007) Electrochemical magneto immunosensing of antibiotic residues in milk. Biosens Bioelectron 22(9):2184–2191
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by National ‘Twelfth Five-Year’ Plan for Science and Technology Support (grant number 2011BAK10B06) and the National Natural Science Foundation of China (grant number 31272604).
Conflict of Interest
Wenhao Tian declares that he has no conflict of interest. Xiaoxiao Zhang declares that she has no conflict of interest. Meirong Song declares that he has no conflict of interest. Haiyang Jiang declares that she has no conflict of interest. Shuangyang Ding declares that she has no conflict of interest. Jianzhong Shen declares that he has no conflict of interest. Jiancheng Li declares that he has no conflict of interest.
Ethical Approval
All applicable China agricultural university laboratory animal management committee guidelines for the care and use of animals were followed.
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Tian, W., Zhang, X., Song, M. et al. An Enzyme-Linked Immunosorbent Assay to Detect Salinomycin Residues Based on Immunomagnetic Bead Clean-up. Food Anal. Methods 10, 3042–3051 (2017). https://doi.org/10.1007/s12161-017-0873-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0873-7