Abstract
The paper presents the analysis of the profile composition of fatty acids in the molecules of phosphatidylcholine and phosphatidylethanolamine, by using hydrophilic interaction liquid chromatography and gas chromatography coupled with mass spectrometry. The profiles of 15 phosphatidylcholine and 8 phosphatidylethanolamine species were analyzed with a newly developed hydrophilic interaction liquid chromatography (HILIC)–electrospray ionization (ESI)–tandem mass spectroscopy (MS/MS) method, by using a new stationary bonded phase. The application of the new method in control and experimental groups of egg yolk revealed significant differences in the composition of phospholipid species containing mainly polyunsaturated fatty acids. Additionally, using GC-MS, the profile of fatty acids in four groups with different dietary supplementation of hens was analyzed and 20 fatty acids in egg yolks were determined. Monounsaturated fatty acids were found in higher amounts than saturated fatty acids and polyunsaturated fatty acids in egg yolks. Oleic acid (18:1) was the major monounsaturated fatty acid in egg yolk while palmitic acid (16:0) was the major saturated fatty acid. Linoleic acid (18:2), arachidonic acid (20:4), and docosahexaenoic acid (22:6) reached the highest levels among the polyunsaturated fatty acids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fats are the most concentrated source of energy in the human diet. They are a source of fatty acids (FAs), including unsaturated essential fatty acids (EFAs), and fat-soluble vitamins A, D, and E. They are also the source from which the body derives components to build the cells, tissues, organs, and materials for the synthesis of some biologically active substances (eicosanoids), in particular, prostaglandins belonging to tissue hormones such as prostacyclin (PGI2) (Fetterman and Zdanowicz 2009). Depending on the presence of double bonds and their number, fatty acid molecules can be divided into the following: saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs). The position of the first double bond counted from the methyl group occurs between series of n-3 (ɷ-3) or n-6 (ɷ-6) acids. FAs of the family ω-3 (α-linolenic acid ALA—C18:3) and ω-6 (linoleic acid LA—C18:2) are not synthesized in the human body and most animals, due to the lack of desaturases which are responsible for introducing the double bond into acid molecule between 3–4 and 6–7 carbon atoms (counting from the methyl group), and therefore must be supplied with food (Behr 1994; Flachs et al. 2009). PUFAs delivered with food may undergo enzymatic transformations that rely on the introduction of further double bonds under the influence of a specific desaturase (Δ6, Δ5) and elongation of the hydrocarbon chain with the participation of elongase enzyme (Fig. 1). Transformation of EFAs takes place in the endoplasmic reticulum of cells, where, in the renaturing and elongation process, they are converted into long-chain LC PUFA—having an essential biological activity (Newton 1996). The sources of PUFAs are plants (Behr 1994), fish (especially cold water fish), clams, oysters, and shrimps (Friedman and Moe 2006), but also plant products, such as nuts (especially English walnuts), sesame seeds (Namiki 2007), and linseed, and vegetable oils such as soybean oil and rapeseed oil (Whelan and Rust 2006).
LC PUFAs are deemed necessary in the human body, because they are essential for the proper development and functioning of the brain and retina. The omega-3 and omega-6 acids are the building components of phospholipidic membrane, and their proportion in the diet to a large extent reflects their relative proportions in the tissues of the body. Long-chain PUFAs are the components of myelin sheath of the peripheral nerves, synaptic membrane, and neurotransmitters (Hibbeln and Salem 1995). Omega-3 with omega-6 acids affects the normal construction and functioning of the skin. Omega-3 fatty acids prevent excessive drying of the skin and prevent its damage. A number of omega-6 acids are responsible for wound healing and epidermis regeneration (Bojarowicz and Wozniak 2008). Eicosapentaenoic acid (EPA) mainly stimulates the cardiovascular system, while docosahexaenoic acid (DHA) is an important structural component of highly active nerve tissue. DHA represents up to 60 % of the total fatty acids in the phospholipids of neurons and plays a key role in the development of the nervous system, occurring during gestation and early childhood. Too low level of DHA in the diet of women will shorten the gestation and cause low birth weight of children (Hansen 1994).
A rich source of phospholipids (PLs) and FAs is hen egg yolk (Ahn et al. 2006). Phosphatidylcholine (PC) and phosphatidylethanolamine (PE) are the main classes of phospholipids found in hen egg yolk. PC is a major natural phospholipid that plays a crucial role in creating and maintaining cell membranes. Moreover, PC is considered to be a source of arachidonic acid (AA) released by phospholipase A2, which is then converted to eicosanoids, including prostaglandins, prostacyclin, and leukotrienes, which are mediators of inflammation (Frega et al. 2012).
The most commonly used techniques for separation of PLs are chromatography techniques (Pari et al. 2016). Hydrophilic interaction liquid chromatography (HILIC) coupled with mass spectrometry (MS) is undoubtedly a helpful analytical tool in assays of complex substances (Buszewski and Noga 2012). Many papers mention the application of HILIC in the separation analysis of PLs. Zhao et al. (2010) proposed a very interesting method of separation and qualitative and quantitative analysis of PC from soy lecithin using TiO2 column in HILIC mode. Holčapek and coworkers determined individual polar and nonpolar lipid classes in human plasma, erythrocytes, and biological tissues using HILIC (Holčapek et al. 2015; Lisa et al. 2011).
The aim of this work was to develop a new method for the separation of phospholipids extracted from egg yolk. For this purpose, a new stationary bonded phase was used, containing two amino groups and alkyl chain C12. Furthermore, hydrophilic interaction liquid chromatography coupled with tandem mass spectrometry (HILIC-MS/MS) was used for the analysis of individual species of PC and PE. This analysis was performed for the control group and the experimental group (by supplementation of diet). Additionally, the effect of hens’ dietary supplementation on the content of PUFAs and the composition of the fatty acid profile of lipid yolk were determined.
Experimental
Chemicals
Acetonitrile and methanol (all LC-MS grade) were purchased from Sigma-Aldrich (Steinheim, Germany). Ultrapure water was obtained from the Milli-Q water system (Millipore, Bedford, MS, USA). The phospholipid standards of PC (≥99 %), PE (≥97 %), lysophosphatidylethanolamine (LPE) (≥97 %), lysophosphatidylcholine (LPC) (≥98 %), sphingomyelin (SM) (≥95 %), phosphatidylglycerol (PG) (≥97 %), and lysophosphatidylglycerol (LPG) (≥97 %) were purchased from Larodan Lipids (Malmö, Sweden). All reagents used in the lab procedure were high-performance liquid chromatography (HPLC) grade; methanol, hexane, and dichloromethane were obtained from Sigma-Aldrich (Steinheim, Germany). Sodium chloride, sulfuric acid, and anhydrous potassium carbonate were obtained from POCh (Gliwice, Poland). Standards for 37 fatty acid methyl ester (FAME) mix (Supelco 37 Component FAME Mix C4-C24) used to establish calibration curves were purchased from Supelco/Sigma-Aldrich (Steinheim, Germany). Helium and argon, purity 99.999 %, were purchased from B.O.C. (Bydgoszcz, Poland).
A 1.5-mg/mL stock solution of all phospholipid standards for HILIC analysis was prepared in pure methanol. Working solutions from 0.2 to 1 mg/mL were obtained by diluting the stock solution with methanol and stored at −20 °C.
Samples
Pure PL fraction from egg yolk (Gladkowski et al. 2011) was obtained from Wroclaw University of Environmental and Life Sciences. Raw eggs were obtained from Japanese quail (Coturnix japonica). The control group of birds were given complete feeding stuff Farm line of G-090 for quail, which included soybean meal, wheat, corn, wheat bran, vegetable oils, calcium carbonate, monocalcium phosphate, sodium chloride, macro and trace elements, vitamins, amino acids, and biologically active compounds. The experimental group received feed Farm line of G-090 enriched with selenium yeast Saccharomyces cerevisiae containing 500 mg/kg selenium and a standard mix of feed enriched with linseed and fish oils, both in the amount of 1 to 6 %, and dried algae in the amount of 0.5 to 1.5 %. The diet was also supplemented by Humokarbowit and Humobentofet formulations in the amount of 0.5 to 2 %, and 0.01 % vitamin E. Four samples from egg yolk were analyzed. The first one was the control group (standard, not enriched diet); the second, third, and fourth were egg yolk from the experimental group (rich diet) in a 5-, 9-, and 13-week consuming diet for laying hens, respectively. More details on the composition of the diet are described by Gladkowski et al. (2014).
HILIC-LC-MS/MS Analysis
Phospholipid separation was performed by liquid chromatography using a homemade alkyl-amide stationary bonded phase that contains two amine groups and organic dodecanoyl chains. It is a modification of a commonly known alkyl-amide packing (Bocian et al. 2015; Buszewski et al. 1994). The stationary phase was synthesized on Kromasil 100 silica gel with specific surface area of 320 m2/g, average pore diameter of 110 Å, and specific pore volume of 0.9 mL/g. Adsorbents were packed into 125 mm × 4.6 mm i.d. stainless steel columns, with the use of the slurry method, by means of laboratory-made equipment and a Haskel (Burbank, CA, USA) packing pump. Approximately 1.5 g of modified silica was made into slurry with 15 mL of chloroform and placed in the packing apparatus. Methanol was used as a packing pressurizing solvent during the filling process. Columns were packed at a constant pressure of 40 MPa. The obtained stationary phases exhibit high carbon content that equals 15.6 %, which provide the surface coverage density of 3 μmol/m2. The application of the support with two amine groups allows to obtain higher coverage density of organic chains as well as better stability in the mobile phase that contains significant amount of water. Due to the composition of hydrophobic and polar groups, this stationary phase offers unique selectivity in the separation of analytes with polar functionalities, e.g., PLs. The structure of the chemically bonded stationary phase is shown in Fig. 2.
The apparatus was a Shimadzu LC-MS 8050 system (Tokyo, Japan). The absorbance was measured at 206 nm using a diode array detector (SPD-M20A). The instrument includes a binary solvent delivery system (LC-30AD), a controller (CBM 20A), an autosampler (SIL-30A), and a column thermostat (CTO-20AC). Instrument control, data acquisition, and processing were performed with LabSolution 5.8 software for HPLC. The injection volume was 1 μL. The column was maintained at 25 °C. Isocratic mobile phase consisted of 80 % acetonitrile and 20 % water. The flow rate was 0.5 mL/min. MS/MS analysis was performed in the positive ionization mode on a triple quadrupole (Shimadzu, Tokyo, Japan) equipped with an electrospray ionization (ESI) source. The ESI settings were as follows: nebulizing gas flow 3 L/min, heating gas flow 10 L/min, temperature of the drying gas 400 °C, DL temperature 250 °C, and interface temperature 300 °C. PL species were monitored in the scheduled multiple reaction monitoring (MRM) mode. Product ion mode m/z 184 was used for identification of PC, and neutral loss mode of 141 was used for identification of PE. Fractions of PC and PE were collected manually to Eppendorf tubes, and each fraction was evaporated using Labconco CentriVap DNA concentrator (Kansas City, USA). Next, the fraction was dissolved in pure methanol. The collected fractions were analyzed using Kinetex C18 column 150 × 4.6 mm with pore size of 100 Å (Phenomenex, Torrance, CA, USA). The flow rate of the mobile phase was 0.4 mL/min, the injection volume was 1 μL, and the separation temperature was 25 °C. Ninety percent acetonitrile and 10 % water were used as a mobile phase.
GC-MS Analysis of Fatty Acids
The GC-MS analysis was performed on 6890N gas chromatograph (Agilent Technologies, Waldbronn, Germany) coupled with mass spectrometer Agilent 5975 Inert XL MSD equipped with Zebron ZB-WAX (30 m length, 0.25 mm film, 0.25 μm internal diameter; Phenomenex, Torrance, CA, USA) capillary column. The oven temperature program was as follows: initially 60 °C held for 2 min, then ramped at 13 °C/min to 150 °C, and then ramped at 2 °C/min to 230 °C and held for 6 min. The temperature of the splitless injector was 240 °C. The MS analyses were carried out in full-scan mode, with scan mass range of m/z 35–450. The spectra were collected at electron ionization (EI) of 70 eV; both ion source and line transfer temperatures were set at 200 °C. FAMEs were identified by comparison of retention times and MS spectra with authentic standards. The acquisition of chromatographic data was performed by using the ChemStation 3 software (Agilent).
Derivatization of Fatty Acids
FAs were methylated according to the modified procedure described by Christie (1984). Ten milligrams of PL fraction from each sample of egg yolk was transferred into the test tube and dissolved in 4 mL of pure methanol. Four milliliters of 1 % solution of sulfuric acid (VI) in methanol was added, and the test tubes were placed in a water bath at 80 °C for 20 min. After cooling, 4 mL of 6 % NaCl was added. The samples were mixed, and the mixture was extracted three times with 4-mL portions of hexane. Hexane extracts were connected and washed with 6 mL of 4 % potassium carbonate solution and subsequently dried. Dried extracts were concentrated by evaporation of the solvent, then dissolved in dichloromethane and analyzed by GC-MS system. Each fraction was analyzed three times.
Statistical Analysis
Significant differences among the investigated groups in terms of the content of ∑ SFA, ∑ MUFA, ∑ PUFA, total ω-3, total ω-6, and ω-6/ω-3 ratio were analyzed by means of ANOVA followed by Newman–Keuls post hoc test (p = 0.05). Additionally, the factor analysis (FA) with Varimax rotation was also utilized to classify the fatty acid composition in different groups of breeds. All the calculation was performed on Statistica software (Statistica ver.7, StatSoft).
Result and Discussion
HILIC-ESI-MS/MS Methods for Determination of Species of PC and PE
In Fig. 3, a chromatogram of egg yolk PLs using a homemade stationary bonded phase in hydrophilic interaction liquid chromatography conditions is presented. The sample components were eluted within 20 min, with the following elution order: PG, LPG, PE, LPE, PC, SM, and LPC. Analysis of PLs from egg yolk using the new stationary phase allows to separate PLs with a polar “head” of the molecule.
Separation of the phospholipid classes in the egg yolk samples: PG phosphatidylglycerol, LPG lysophosphatidylglycerol, PE phosphatidylethanolamine, LPE lysophosphatidylethanolamine, PC phosphatidylcholine, SM sphingomyelin, LPC lysophosphatidylcholine. Mobile phases are 80 % acetonitrile and 20 % water; flow rate 0.5 mL/min
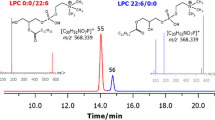
The new stationary phase has a good selectivity in the designation of PLs. It is believed that the structure of the stationary phase (amino groups and the dodecanoyl chain) determines good selectivity against the analyzed compounds. Compared with other works (Anesi and Guella 2015; Schwalbe-Herrmann et al. 2010; Viidanoja 2015), in which the HILIC chromatography was used, the PLs were separated isocratically. Analysis of the hydrophilic interaction liquid chromatography–electrospray ionization–tandem mass spectroscopy (HILIC-ESI-MS/MS) was used to determine the fatty acid composition of the molecules of PC and PE (fractions 3 and 5, respectively, in Fig. 3). Fractions of PC and PE were separated using C18 column. The separation mechanism of PC and PE on the octadecyl stationary phase was based on hydrophobic interactions. Lipids which are more hydrophobic are strongly retained and eluted later from the column. Elution order of PLs in reversed phase depends on the differences in the chain lengths and the number of double bonds of acyl residues. The elution order of lipids in reversed phase is presented using the coefficient equivalent carbon number (ECN) for fatty acid (Perona and Ruiz-Gutierrez 2003). ECN is defined as ECN = CN − 2n (where CN is the sum of the total carbon atom number of fatty acyl groups and n is the number of the sum of double bonds). The retention time of phospholipids increases proportionally to the ECN coefficient (Table 1). PC and PE are the predominant components of PLs in egg yolk. Due to the fact that egg is a complex biological matrix, it is very hard to separate the combination of FAs. In order to determine the molecule PC and PE, a specific scan mode MRM using positive ESI coupled to MS/MS was applied, since these lipids lose their headgroups during collision-induced dissociation (CID). PC can be identified by the appearance of the spectrum in positive ion mode with the value of fragment m/z 184, derived from phosphocholine headgroup C5H15NO4P+. In the case of PE, a loss of neutral molecule m/z 141 corresponds to the loss of the polar head C2H9NO4P+ (phosphoethanolamine) (Brugger et al. 1997). Table 1 presents all identified PUFAs contained in PC and PE for the two groups of egg yolks. In this study, we have found the following phospholipids, PC 44:12, PC 38:7, PC 38:6, PC 38:4, PC 36:6, PE 38:6, and PE 38:5, which occur in the experimental group (13 weeks of consuming the diet for laying hens), and they are not present in the control group.
GC-MS Analysis of Fatty Acids
The total relative fatty acid profile was analyzed in the egg yolk samples using GC-MS. Since the metabolism of FAs is dependent on the relative cellular fatty acid composition, rather than the absolute concentrations, we focused on the comparison of fatty acid profiles in the four studied groups. As shown in Tables 2, 3, 4, and 5, we were able to verify the results obtained with the metabolomic approach by targeted GC-MS analysis.
Individual and total saturated fatty acid concentrations are given in Table 2. In the test samples of eggs, saturated fatty acids constitute about 44.80 % of the total amount of FAs. This percentage was at the same level for group II but had significantly decreased for groups III and IV. The most abundant of saturated fatty acids was palmitic acid, constituting about 31.37 % of the total fatty acid composition. The highest value of palmitic acid was recorded for experimental group number II. FAs with an odd number of carbon atoms in the molecule represented only 0.14 % of the total FA composition.
The composition of monounsaturated fatty acids is presented in Table 3. The MUFA content was at the same level for groups I and II and significantly decreased for groups III and IV. The highest value of palmitoleic acid (C16:1) was recorded in the control group followed by experimental groups III and IV, while lowest concentration was recorded for II group (Table 3). Oleic acid, with an average content of 39.10 %, dominated in the overall composition.
Polyunsaturated fatty acids play a crucial role among unsaturated acids mainly due to their physiological functions. The total content of PUFA was significantly different between all investigated groups. Among the PUFAs, the most abundant acid was linoleic acid, which constituted 9.54 % of the total amount of polyunsaturated fatty acids (Table 4). The biggest difference in fatty acid composition was determined for α-linolenic acid (ALA), which increased to 0.05 % in the experimental group III. The contents of ALA and the DHA were 0.05 and 0.52 %, respectively, in the control group of PL fraction, but the presence of only DHA (2.39, 3.56, and 2.07 %) was observed in the experimental groups. This phenomenon can be explained by the metabolic pathway of PUFA, wherein DHA is synthesized from ALA by the elongation and desaturation of the hydrocarbon chain (Fig. 1). The higher content of DHA in the experimental group compared to the control group is the result of the metabolism of ALA and the ingested diet. In the case of EPA, it is formed by biosynthesis by enzymatic interconversion of ALA, which occurs (to a very limited degree) in the human body. At this stage, there are several steps of the fatty acid chain elongation, i.e., the step of lengthening the carbon chain of two carbon atoms, and the desaturation step, in which the fatty acid molecule produces next unsaturated bonds. These transformations are catalyzed by enzymes Δ6 desaturase and Δ5 and elongase (Fig. 1). The highest content of AA (C20: 4) was observed for the control group. This acid is biosynthesized in vivo from LA. The highest content of LA was detected in the control group without supplementation of the diet; therefore, the content of AA was significantly higher in the control group compared to the experimental group. Gamma-linolenic acid (GLA, C18:3) was determined only for the control group at the level of 0.05 %. GLA is produced from linoleic acid. The mixture of such enriched feed added to hens’ diet forced the reduction in the content of monounsaturated fatty acids in raw egg.
Feeding hens with enriched feed had a significant effect on the ratio of ω-6/ω-3 of fatty acids, significantly reducing the value of 25.72 % for the test group and below 4 % for other groups (Table 5). Fat in eggs of quails fed with basic diet contained significantly less essential fatty acids from the omega-3 group.
Factor analysis has been performed in the range of all investigated fatty acids (20 parameters). Three repetitions of each group were used as cases. Three factors with eigenvalue bigger than 1 explained 97 % of total variance of data and grouped the cases into four previously defined classes (Fig. 4).
Samples from group I were characterized with the highest scores of factor 1 that corresponds to the high content of C14:1, C16:0, C17:0, C18:1 n-9, C18:2 n-6, C20:1, C20:3 n-6, and C20:3 n-3 fatty acids. Group II with elevated scores of factor 3 had the lowest content of C17:1 fatty acid while distinguishing between cases from groups III and IV was possible through scores of factor 2 positively correlated with amounts of C20:5 n-3, C22:5 n-6, and C22:6 n-3 fatty acids.
The increase in the amount of linseed oil and the reduction in marine fish oil in the diet cause a significant decrease in saturated fatty acids and an increase in essential fatty acids of the n-3 family in egg yolk. Many authors conclude that hens have the ability to synthesize EPA and DHA from linoleic acid if they receive it in sufficient amounts in the diet (Kralik et al. 2008). Literature reports that egg yolk is a rich source of EFAs of the n-3 and n-6 acid family, the amount of which depends mainly on the genotype and age of laying hens (Polat et al. 2013). Bean and Leeson show that there is no effect of hens’ origin on the level of ALA (C18:3) in the egg yolk (Bean and Leeson 2003). The recommended intake of omega-3 is 0.5–2 % of the total energy supplied. According to the recommendation of the American Heart Association, adults should eat meals composed of fish that are high in fat, at least twice a week; for patients with coronary heart disease, a daily intake 1 g of EPA and DHA is recommended (Block and Pearson 2006). According to the recommendations of the International Society for the Study of Fatty Acids and Lipids, the amount of energy from the intake of LA should be 2 %, and in the case of ALA it should be 0.7 % (Sanders 2000). The results of several studies suggest that the value of the omega-6/omega-3 ratio should be low, and the approximately 4–5:1 ratio of omega-6 to omega-3 fatty acids is considered as most favorable. In addition, it is believed that the high ratio of omega-6 to omega-3 acids in the diet promotes the development of cardiovascular diseases, cancer, inflammatory diseases, and autoimmune diseases (Simopoulos 2008).
Conclusion
The GC-MS methodology is a powerful tool for exploring differences at the metabolite level. Furthermore, we developed a sensitive and reliable HILIC-MS/MS method for the simultaneous qualitative analysis of 15 PC and 8 PE species, containing polyunsaturated fatty acids in two groups of egg yolk. Additionally, a new stationary bonded phase was applied, which demonstrated good separation of phospholipid classes in a short time. The results indicate the possibility of enriching quail eggs with polyunsaturated fatty acids, in particular EPA and DHA, by using feed containing selenium yeast, algae, and flaxseed for feeding poultry. The explanation of the mechanism of polyunsaturated fatty acids allows properly balanced diet in order to achieve good health and planned prophylactic effect. When using feed supplementation, an increase in n-3 PUFA and a reduction in ω-6/ω-3 ratio in egg yolk lipids were observed.
References
Ahn DU, Lee SH, Singam H, Lee EJ, Kim JC (2006) Sequential separation of main components from chicken egg yolk. Food Sci Biotechnol 15:189–195
Anesi A, Guella G (2015) A fast liquid chromatography-mass spectrometry methodology for membrane lipid profiling through hydrophilic interaction liquid chromatography. J Chromatogr A 1384:44–52
Bean LD, Leeson S (2003) Long-term effects of feeding flaxseed on performance and egg fatty acid composition of brown and white hens. Poult Sci 82:388–394
Behr SR (1994) Effects of fish oil and vegetable oil formula on platelet aggregation and neutrophils. Am J Clin Nutr 5:717
Block RC, Pearson TA (2006) The cardiovascular implications of omega-3 fatty acids. Cardiol J 13:557–569
Bocian S, Dziubakiewicz E, Buszewski B (2015) Influence of the charge distribution on the stationary phases zeta potential. J Sep Sci 38:2625–2629
Bojarowicz H, Wozniak B (2008) Wielonienasycone kwasy tłuszczowe oraz ich wpływ na skórę. Prob Hig Epidemiol 89(4):471–475
Brugger B, Erben G, Sandhoff R, Wieland FT, Lehmann WD (1997) Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proc Natl AcadSci USA 94:2339–2344
Buszewski B, Noga S (2012) Hydrophilic interaction liquid chromatography (HILIC)—a powerful separation technique. Anal Bioanal Chem 402(1):231–247
Buszewski B, Jaroniec M, Gilpin RK (1994) Influence of eluent composition on retention and selectivity of alkylamide phases under reversed-phase conditions. J Chromatogr A 668:293–299
Christie WW (1984) In: K. MH (ed) Handbook of chromatography, vol. 1. CRC Press, Boca Raton, pp. 33–46
Fetterman JW, Zdanowicz MM (2009) Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am J Health Syst Pharm 66(13):1169–1179
Flachs P, Rossmeisl M, Bryhn M, Kopecky J (2009) Cellular and molecular effects of n−3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin Sci 116:1–16
Frega NG, Pacetti D, Boselli E (2012) Characterization of phospholipid molecular species by means of HPLC-tandem mass spectrometry—applications and principles. INTECH 27:637–672
Friedman A, Moe S (2006) Review of the effects of omega-3 supplementation in dialysis patients. Clin J Am Soc Nephrol 1(2):182–192
Gladkowski W, Kiełbowicz G, Chojnacka A, Bobak Ł, Spychaj R, Dobrzański Z, Trziszka T, Wawrzeńczyk C (2014) The effect of feed supplementation with dietary sources of n-3 polyunsaturated fatty acids, flaxseed and algae Schizochytrium sp., on their incorporation into lipid fractions of Japanese quail eggs. Int J Food Sci Tech 49(8):1876–1885
Gladkowski W, Kiełbowicz G, Chojnacka A, Gil M, Trziszka T, Dobrzański Z, Wawrzeńczyk C (2011) Fatty acid composition of egg yolk phospholipids fractions following feed supplementation of Lohmann Brown hens humic-fat preparations. Food Chem 126:1013–1018
Hansen HS (1994) New biological roles of omega 6 and omega 3 fatty acids. Nutr Rev 5:162
Hibbeln JR, Salem M (1995) Dietary PUFAs and depression. Am J Clin Butr 1:42
Holčapek M, Cifkova E, Cervena B, Lisa M, Vostalova J, Galuszka J (2015) Determination of nonpolar and polar lipid classes in human plasma, erythrocytes and plasma lipoprotein fractions using ultrahigh-performance liquid chromatography-mass spectrometry. J Chromatogr A 1377:85–91
Kralik G, Skrtic Z, Suchy P, Strakova E, Gajcevic Z (2008) Feeding fish oil to laying hens to increase the n-3 PUFA of egg yolk. Acta Vet Brno 77:561–568
Lisa M, Cifkova E, Holčapek M (2011) Lipidomic profiling of biological tissues using off-line two-dimensional high-performance liquid chromatography–mass spectrometry. J Chromatogr A 1218:5146–5156
Namiki M (2007) Nutraceutical functions of sesame: a review. Crit Rev Food Sci Nutr 47:651–673
Newton JS (1996) Long chain fatty acids in health and nutrition. J Food Lipids 3:233–249
Pari S, Nie B, Arnold RD, Cummings BS (2016) Extraction, chromatographic and mass spectrometric methods for lipid analysis. Biomed Chromatogr 30:695–709
Perona JS, Ruiz-Gutierrez V (2003) Simultaneous determination of molecular species of monoacylglycerols, diacylglycerols and triacylglycerols in human very low density lipoproteins by reversed phase liquid chromatography. J Chromatogr B 785(1):89–99
Polat ES, Citil OB, Garip M (2013) Fatty acid composition of yolk of nine poultry species kept in their natural environment. Anim Sci Pap Rep 31:363–368
Sanders TAB (2000) Polyunsaturated fatty acids in the food chain in the food chain in Europe. Am J Clin Nutr 71:176–178
Schwalbe-Herrmann M, Willmann J, Leibfritz D (2010) Separation of phospholipid classes by hydrophilic interaction chromatography detected by electrospray ionization mass spectrometry. J Chromatogr A 1217:5179–5183
Simopoulos AP (2008) The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med 233:674–688
Viidanoja J (2015) Analysis of phospholipids in bio-oils and fats by hydrophilic interaction liquid chromatography–tandem mass spectrometry. J Chromatogr B 1001:140–149
Whelan J, Rust C (2006) Innovative dietary sources of n-3 fatty acids. Ann. Rev Nutr 26:75–103
Zhao J, Jiang Z-T, G-R L, Tan J, Li R (2010) Determination of phosphatidylcholine in soybean lecithin samples by high performance liquid chromatography on titania. Anal Chem 2:1779–1783
Acknowledgments
This work was supported by the National Science Center (Cracow, Poland), no. NCN 2013/11/N/ST4/01838 (for period 2014–2016), NCBiR no. BIOSTRATEG-II/389/2015 (for period 2016–2019), Faculty of Chemistry NCU grant for young scientists nos. 2084-Ch and 2354-Ch, budget of Kuyavia and Pomerania and the European Regional Development Fund under the ROP for the years 2007–2013, project no. RPKP.05.04.00-04-003/13, and “Krok w przyszłość” (Step into the Future) Sectoral Operational Program—Human Resources, 2015–2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human or animal subjects.
Informed Consent
Informed consent was not applicable.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Walczak, J., Bocian, S., Kowalkowski, T. et al. Determination of Omega Fatty Acid Profiles in Egg Yolk by HILIC-LC-MS and GC-MS. Food Anal. Methods 10, 1264–1272 (2017). https://doi.org/10.1007/s12161-016-0655-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0655-7