Abstract
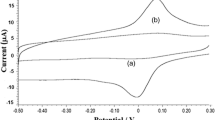
A sensitive voltammetric method for the determination of tert-butylhydroquinone (TBHQ), a widely used synthetic phenolic antioxidant in oils and fats, using multiwalled carbon nanotube modified gold electrode (MWCNT/GE) was developed. In 0.10 M phosphate buffer solution (PBS) of pH 2, TBHQ gave redox peaks at E pa = 258 mV and E pc = 228 mV on MWCNT/GE. Diffusion-controlled electrooxidation of TBHQ was found to be perfectly reversible with the involvement of two electrons and two protons. The anodic peak currents varied linearly with concentrations of TBHQ in the range 4.0 × 10−6 to 1.00 × 10−4 M. The limit of detection achieved for the developed sensor was 3.20 × 10−8 M (5.31 ng mL−1). Developed sensor was used for the determination of TBHQ in commercially available coconut oil. The results obtained from the developed method were in good agreement with the standard method (HPLC-UV).







Similar content being viewed by others
References
Andrea C, Castanheira I, Cruz JM, Paseiro P, Silva A (2010) Analytical strategies to evaluate antioxidants in food. Trends Food Sci Technol 21:229–246
Anson F, Huang WZ, Gao XX (1983) Electrochemistry and electroanalytical chemistry. Beijing University Press, Beijing
Araujo TA, Barbosa AMJ, Viana LH, Ferreira VS (2011) Electroanalytical determination of TBHQ, a synthetic antioxidant, in soybean biodiesel samples. Fuel 90:707–712
Balasubramanian K, Burghard M (2008) Electrochemically functionalized carbon nanotubes for device applications. J Math Chem 18:3071–3083
Bond AM (1980) Modern polarographic methods in analytical chemistry. Marcel Dekker, New York
Britto PJ, Santhanam KSV, Ajayan PM (1996) Carbon nanotube electrode for oxidation of dopamine. Bioelectrochem Bioenerg 41:121–125
Caramit RP, Andrade AGF, Souza JBG, Araujo TA, Viana LH, Trindade MAG, Ferreira VS (2013) A new voltammetric method for the simultaneous determination of the antioxidants TBHQ and BHA in biodiesel using multi walled carbon nanotube screen printed electrodes. Fuel 105:306–313
Chandran S, Lonappan LA, Thomas D, Jos T, Kumar KG (2014) Development of an electrochemical sensor for the determination of amaranth: a synthetic dye in soft drinks. Food Anal Methods 7:741–746
Cortes AG, Armisen P, Ruiz MA, Sedeno PY, Pingarron JM (1994) Electroanalytical study of the antioxidant tert-butylhydroquinone in an oil in water emulsified medium. Electroanalysis 6:1014–1019
Crompton TR (1979) Additive migration from plastics into food. Pergamon Press, London
De la Fuente C, Acuna JA, Vazquez MD, Tascon LM, Batanero PS (1999) Voltammetric determination of the phenolic antioxidants 3-tert-butyl-4-hydroxyanisole and tert-butylhydroquinone at a polypyrrole electrode modified with a nickel phthalocyanine complex. Talanta 49:441–452
Gan T, Sun J, Cao S, Gao F, Zhang Y, Yang Y (2012) One step electrochemical approach for the preparation of graphene wrapped-phosphotungstic acid hybrid and its application for simultaneous determination of sunset yellow and tartrazine. Electrochim Acta 74:151–157
Gharavi N, Haggarty S, Ayman OS (2007) Chemoprotective and carcinogenic effects of tert-butylhydroquinone and its metabolites. Curr Drug Metab 8:1–7
Ghoreishi SM, Behpour M, Golestaneh M (2012) Simultaneous determination of sunset yellow and tartrazine in soft drinks using gold nanoparticles carbon paste electrode. Food Chem 132:637–641
Gonzalez M, Ballesteros E, Gallego M, Varcarcel M (1998) Continuous flow determination of natural and synthetic antioxidants in foods by gas chromatography. Anal Chim Acta 359:47–55
Goulart LA, Teixeira ARL, Ramalho DA, Terezo AJ, Castilho M (2014) Development of an analytical method for the determination of tert-butylhydroquinone in soybean biodiesel. Fuel 115:126–131
Gryger T, Marken F, Schroder U, Scholz F (2002) Electrochemical analysis of solids. Collect Czechoslov Chem Commun 67:163–208
Guan Y, Chu Q, Fu L, Wu T, Ye J (2006) Determination of phenolic antioxidants by micellar electrokinetic capillary chromatography with electrochemical detection. Food Chem 94:157–162
Guo L, Xie MY, Yan AP, Wan YQ, Wu YM (2006) Simultaneous determination of five synthetic antioxidants in edible vegetable oil by GC–MS. Anal Bioanal Chem 386:1881–1887
Issac S, Kumar KG (2009) Voltammetric determination of sulfamethoxazole at a multiwalled carbon nanotube modified glassy carbon sensor and its application studies. Drug Test Anal 1:350–354
Jos T, Issac S, Joseph R, Rajith L, Kumar KG (2012) Electrocatalysis and determination of pyridine-2-aldoxime methochloride using carbon nanotube modified gold electrode. Micro Nano Lett 7:854–858
Li XQ, Ji C, Sun YY, Li YM, Chu XG (2009) Analysis of synthetic antioxidants and preservatives in edible vegetable oil by HPLC/TOF-MS. Food Chem 113:692–700
Lin X, Ni Y, Kokot S (2013) Glassy carbon electrodes modified with gold nanoparticles for the simultaneous determination of three food antioxidants. Anal Chim Acta 765:54–62
Lonappan L, Issac S, Joseph R, Thomas D, Kumar KG (2011) Electrochemical studies of TAM using multiwalled carbon nanotube modified glassy carbon sensor. Micro Nano Lett 6:867–870
Randles JEB (1948) Cathode ray polarograph. Trans Faraday Soc 44:322–327
Rasheed Z, Vikraman AE, Thomas D, Jagan JS, Kumar KG (2014) Carbon nanotube based sensor for the determination of butylated hydroxyanisole in food samples. Food Anal Methods. doi:10.1007/s12161-014-9894-7
Robards K, Dilli S (1987) Analytical chemistry of synthetic food antioxidants. Analyst 112:933–943
Saad B, Sing YY, Nawi MA, Hashim N, Ali ASM, Saleh MI (2007) Determination of synthetic phenolic antioxidants in food items using reversed-phase HPLC. Food Chem 105:389–394
Shahidi F (2000) Antioxidants in food and food antioxidants. Nahrung 44:158–163
Summary of evaluations performed by the Joint FAO/WHO Expert Committee on Food Additives (JECFA 1956–2007). [http://jecfa.ilsi.org/index.htm, www.efsa.europa.eu, www.fao.org]
Tagliabue S, Gasparoli A, Bella DL, Bondioli P (2004) Quali-quantitative determination of synthetic antioxidants in biodiesel. Riv Ital Sostanze Grasse 80:37–40
Thomas D, Rajith L, Lonappan L, Issac S, Kumar KG (2012) Sensitive determination of nitrite in food samples. Food Anal Methods 5:752–758
Tsang SC, Chen YK, Harris PJE, Green MLH (1994) A simple chemical method of opening and filling carbon nanotubes. Nature 372:159–161
Vikraman AE, Rasheed Z, Rajith L, Lonappan LA, Kumar KG (2013) MWCNT-modified gold electrode sensor for the determination of propyl gallate in vegetable oils. Food Anal Methods 6:775–780
Wang MY, Zhang DE, Tong ZW, Xu XY, Yang XJ (2011) Voltammetric behavior and the determination of quercetin at a flowerlike Co3O4 nanoparticles modified glassy carbon electrode. J Appl Electrochem 41:189–196
Xu JZ, Zhu JJ, Wu Q, Hu Z, Zhen HY (2003) An amperometric biosensor based on the coimmobilisation of horseradish peroxidase and methylene blue on a carbon nanotubes modified electrode. Electroanalysis 15:219–224
Zhao Q, Gan Z, Zhuang Q (2002) Electrochemical sensors based on carbon nanotubes. Electroanalysis 14:1609–1613
Acknowledgments
The authors are grateful to the Defence Research and Development Organisation (DRDO), the Government of India for the financial assistance in the form of a research project to carry out this work. Krishnapillai Girish Kumar has received research grant from DRDO, Government of India.
Conflict of interest
Ambily Thomas declares that she has no conflict of interest. Anuja Elevathoor Vikraman declares that she has no conflict of interest. Divya Thomas declares that she has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thomas, A., Vikraman, A.E., Thomas, D. et al. Voltammetric Sensor for the Determination of TBHQ in Coconut Oil. Food Anal. Methods 8, 2028–2034 (2015). https://doi.org/10.1007/s12161-015-0092-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0092-z