Abstract
Chromatographic system for simultaneous determination of levodopa, biogenic amines, and methylxanthines in food has been developed. Chromatographic column and pre-column with octadecylsilane phase and simultaneously fluorescence and DAD detectors have been used. Gradient elution with acetate buffer (pH = 4.66) with acetonitrile has been applied. Examination included levodopa, norepinephrine, dopamine, normetanephrine, tyramine, and serotonin as well as caffeine, theophylline, and theobromine. Limit of detection (LOD) and limit of quantitation (LOQ) have been determined for all compounds with signal to noise ratio (S/N) equal to 3 and 10, respectively. LOD of 10 ng/mL and LOQ of 30 ng/mL for levodopa and biogenic amines as well as LOD of 70 (60) ng/mL and LOQ of 210 (180) ng/mL for methylxanthines have been determined. Authors have also developed method for simultaneous separation of all analytes from food matrix. Developed chromatographic system with sample preparation method has been applied for determination of examined compounds in cocoa products, vegetables, and fruits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biogenic amines are formed as a result of decarboxylation of amino acids. Those compounds can be divided to polyamines, catecholamines, imidazole, and indoleamines. Biogenic amines are responsible for cellular processes, generated in the brain and used by human body as neurotransmitters. A group of catecholamines include, among others, tyramine, dopamine, epinephrine, norepinephrine, and normetanephrine. Group of heterocyclic indoloamines include, among others, serotonin.
Determination of biogenic amines in food is important because of their influence on humans, especially because serotonin or tyramine could be toxic in higher concentration. Tipton (2002) reported that consumer products rich in amines can cause migraine headache in people. Tyramine is presser amine which can cause peripheral vasoconstriction and salivation, increases respiration and blood sugar level, and releases noradrenaline (Shalaby 1996). Tyramine is present plentifully in vegetables as well as in animal foodstuffs (matured cheeses (Yigit and Ersoy 2003; Vale and Glória 1998), chocolate (Bruinsma and Tarner 1999; Pastore et al. 2005), red meat (Vinci and Antonelli 2002)). Dopamine is synthesized and released by neurons of central nervous system. Its role depends on the place of its activity. Dopamine responds for body kinetics, muscle coordination and tonus, emotions, and appetite control. One of Parkinson’s disease (PD) symptoms is deficiency of dopamine in extrapyramidal system. Dopamine occurs in vines (Hlabangana et al. 2006), bananas (Lavizzari et al. 2006), as well as chocolate (Pastore et al. 2005) and cocoa products (Melzig et al. 2000). Serotonin controls blood pressure (vasoactive compound). It is responsible for smooth muscle stimulation and sensory threshold increase as well as inhibits gastric secretion (Halász and Baráth 2002). Serotonin can be found in meat products (Bruinsma and Tarner 1999) as well as vegetables (banana (Adão and Glória 2005)), oranges, cocoa, chocolate (Pastore et al. 2005), and coffee (Ciriloa et al. 2003; Vasconcelos et al. 2007).
Levodopa is a dopamine precursor. Thanks to its ability to penetrate blood-brain barrier, levodopa has been found useful in Parkinson’s disease therapy. Main advantage of levodopa is the reduction of symptoms of PD; however, it has no impact on speed of degradation of locus niger (substantia nigra) (Biagio Mercuri and Bernardi 2005). Levodopa is present in vegetables from Leguminosae family, like broad been, lentil (Chen et al. 2005), and Mucuna pruriens (Siddhuraju and Becker 2001).
Methylxanthines are purines alkaloids which contain nitrogen atoms in heterocyclic configurations. Methylxanthines include, among others, caffeine (1,3,7-trimethylxanthine), theophylline (1,3-dimethylxanthine), and theobromine (3,7-dimethylxanthine). Caffeine is widely known for its properties such as stimulation of the central nervous system and cardiac stimulation. However, the consumption of high quantity of this compound can cause some undesirable side effects such as cardiac arrhythmia, excitement, nausea, and gastric acidity. Caffeine, which is not a neurotransmitter, is an antagonist of adenosine receptors. When caffeine is taken up in the brain, caffeine combines with adenosine receptors and excludes adenosine, and therefore, this activates dopamine receptors (Nishi et al. 2010). Theophylline and theobromine are responsible for central nervous system stimulation. Moderate concentration of those compounds in human body stimulates serotonin release in cerebral cortex and cerebellum. Methylxanthines in high concentration have side effects to human comfort—can lead to unrest, irritation, or insomnia. Methylxanthines occur in coffee, tea (Hicks et al. 1996), yerba mate (Isolabella et al. 2010), and chocolate (Bruinsma and Tarner 1999; Matissek 1997); thus, they are widely present in human diet.
Biogenic amines together with methylxanthines are present in such products like cocoa, chocolate (Bruinsma and Tarner 1999; Pastore et al. 2005), tea (Higashimoto et al. 2000), and coffee (Ciriloa et al. 2003; Oliveira et al. 2005). It should be emphasized that until now, there have been no publicized records of determination of all examined compounds in the mentioned food products.
Chemical structure of tyramine, dopamine, as well as serotonin allows one to determine its concentration with the use of liquid chromatography using fluorescence (Baranowska and Płonka 2008; Baranowska and Zydroń 2002; Ciriloa et al. 2003; Vasconcelos et al. 2007; Zydroń et al. 2005), spectrophotometric (Romero et al. 2000), and electrochemical (Pastore et al. 2005; Higashimoto et al. 2000; Yashin and Yashin 2004; Zydroń et al. 2005) detectors. For methylxanthines, diode array detector (DAD) (Baranowska and Zydroń 2002; Baranowska et al. 2006; Hicks et al. 1996; Matissek 1997; Zydroń et al. 2004) is widely used. Interest in application of ultra high-performance liquid chromatography has increased in the last few years (Tzanavarasa et al. 2010; Dadáková et al. 2009; Mayer et al. 2010; Latorre-Moratalla et al. 2009). Such method allows one to significantly shorten time of chromatographic analysis. However, no chromatographic procedures for simultaneous determination for biogenic amines together with methylxanthines with the use of HPLC has been developed neither.
The fundamental problem is the development of sample preparation procedures for simultaneous extraction of analytes from food samples. Variety and specificity of food sample require many stages of initial preparation like homogenization, degassing, and degreasing. For biogenic amines, extraction procedures solid-phase extraction (Hernández-Orte et al. 2008), liquid-liquid extraction (Lavizzari et al. 2006) and solid-liquid extraction were used. Sometimes solid-liquid extraction was supported by ultrasound (Pastore et al. 2005; Favaro et al. 2007; Tang et al. 2006). Additionally, Calbiani et al. (2005) and Chiacchierini et al. (2006) examined the possibility to apply matrix solid-phase dispersion for tomatoes and cheese samples. Even though authors analyzed variety of biogenic amines, not every procedure was suitable for simultaneous extraction of every analyte from samples. Different solvents were proposed for extraction of aliphatic amines, other for catecholamines and for heterocyclic amines.
For methylxanthines, extraction process was supported by temperature increase or by use of Soxhlet apparatus. Effectivity of methylxanthines extraction from food matrix with the use of particular solvent depended not only on the analyte-solvent affinity but also on the presence of other compounds in the matrix. Caudle et al. (2001) stated that several acids present in maté bind caffeine into complexes; thus, extraction effectivity of caffeine from leafs is much worse than from guarana seeds for the same process parameters. The lack of additional stage of purifying has a positive influence on the obtained recoveries of analytes. However, it could cause matrix interference.
Expanding consumers’ demands require food quality control to be improved all the time. The examination of amines concentration in food is particularly interesting because they can be used as biosensors (McCabe-Sellers et al. 2006; Roig 2002). It is very important to obtain as much data as possible in one analysis, which means simultaneous determination of compounds from more than one group and opportunity to use one procedure for several different food products.
In the current work, chromatographic method as well as sample preparation procedure for examination of selected biogenic amines and methylxanthines mixture was developed. New procedures for food sample preparation were planned to allow one for simultaneous extract and enrich dozen of analytes as well as remove matrix effect from real sample. Consequently, procedures for complex food product analysis were created, which can be used in food processing.
Materials and Methods
Reagents
Standard solutions (1 mg/mL) in 0.1 M HCl with Na2S2O5 (5 g/L) from levodopa (l-DOPA), dopamine (DA), norepinephrine (NE), normetanephrine (NMN), tyramine (TYR), and serotonin (5HT) (Sigma-Aldrich) were prepared. Standard solutions (1 mg/mL) in methanol from caffeine (CAFF) and theophylline (THPH) and in water from theobromine (THBR) (Sigma-Aldrich) were prepared. Acetonitryl, water, methanol, and acetate buffer (pH = 4.66) of HPLC grade used in this work for mobile phase preparation were purchased from Merck, Darmstadt, Germany.
High-Performance Liquid Chromatography
HPLC analyses were performed using a Merck-Hitachi chromatograph equipped with a L6200A pump, an L-7480 fluorescent (FL), and L-4500A DAD. Chromatographic separations were carried out on a LiChroCARD Purospher column RP-18e, 125–3 mm, 5-μm particle size accompanied with a LiChroCARD 4–4-mm pre-column packed with LiChrospher 100 RP-18e, 5-μm particle size (Merck, Germany) at a room temperature. Gradient elution with acetate buffer (pH = 4.66) (A) and methanol (B) was applied. The gradient applied was programmed as follows: 0 min 100 % A and flow rate of 0.5 ml/min, 30 min 90 % A, 10 % B and flow rate of 1.0 ml/min. Detection for biogenic amines was performed with the use of fluorescent detector at excitation wavelength of λ EX = 285 nm and emission wavelength of λ EM = 315 nm. Detection for methylxanthines was performed with the use of fluorescent detector (λ EX = 285 nm, λ EM = 315 nm) as well as DAD detector, where the wavelength was set to λ = 275 nm. Developed chromatographic procedure does not require prior derivatization of analytes.
Calibration Curves
A calibration curve for determined compounds in cocoa and broad beans matrix was prepared. Calibration curve for levodopa and biogenic amines was prepared for solutions in the range of 0.06–3 μg/mL and 2–100 μg/mL for methylxanthines. The number of experimental points taken for regression was n = 6. Every analyte was injected thrice. The volume of the solution in every single injection was 20 μL. Limit of detection (LOD) and limit of quantitation (LOQ) have been determined.
Sample Preparation
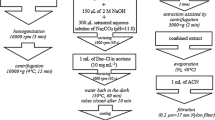
Samples of chocolate with 90 % cocoa, cocoa, broad bean, and bean were examined in this work. Chocolate was grated to shavings, broad bean was pared and cut into small pieces, and bean was cut into small pieces. Ten grams of each product was weighted and poured with 50 mL of perchloric acid (0.2 M). Samples were precisely intermixed and left for 24 h. Those mixtures were shaken from time to time. After this time, samples were centrifuged for 20 min (4,000 rpm). The examined material was filtrated using a Bakerbond nylon filter with 0.2-μm porous. The volume of the filtrated solution in every single injection was 20 μL. Matrix for standards which was used for calibration curves was prepared analogous to samples. The analytes were removed through extraction and then the samples were treated with new portion of solution (0.2 M HClO4). After carrying out the above procedure, the matrix of the standards for calibration curves was obtained.
Results and Discussion
Developed chromatographic system allows one to separate and determine levodopa and five biogenic amines: NE, DA, NMN, TYR, and 5HT together with CAFF, THPH, and THBR. Basing on the performed examination, it was found that the best separation of biogenic amines and methylxanthines was obtained by the use of RP-18e column. It was confirmed in the literature review, where authors in their research have been using C18e stationary phases. Chromatographic elution was performed with the use of gradient system of acetic buffer (pH = 4.66) with acetonitrile.
The total time of every single assay has not exceeded 30 min. Chromatogram of mixture of biogenic amines and methylxanthines standards on chocolate matrix is shown on Fig. 1a. This chromatogram was recorded with fluorescence detector. The chromatogram of the same mixture recorded with diode array detector with a wavelength of 275 nm is shown on Fig. 1b. As it can be seen, all examined compounds were well separated. Peaks from biogenic amines could not be observed on chromatograms from DAD because concentration of biogenic amines in solution was too small to record them with the use of this detector. Peaks from methylxanthines are sharply outlined. Simultaneously, peaks of high intensity of both biogenic amines and methylxanthines could be observed on chromatogram recorded with fluorescence detector (Fig. 1a).
Large advantage of the developed chromatographic system is a lack of derivatization stage of catecholamines and indolamine. This step is often mentioned to be used in the analysis of the biogenic amines, but it cannot be enough selective and can introduce additional interferences from the matrix.
The use of 0.05 % trifluoroacetic acid (TFA) solution in the water was examined; however, retention times were rendered longer and no signals for methylxanthines was recorded with fluorescence detector. The examination of pH impact on the ability to record those signals was performed. The use of the mixtures of citrate buffer with pH = 2.0 (which is comparable to pH of 0.05 % TFA solution) with acetonitrile and TFA water solution with pH 5.0 with acetonitrile rendered intensity of fluorescence signals of methylxanthines to be lower.
Retention times of each analytes, calibration curve parameters, and limits of detection and quantification from both fluorescence and DAD detectors are presented in Table 1. Data contents in part “a” of the table come from the fluorescence detector, whereas part “b” of this table listed data from the DAD detector. The retention times of methylxanthines recorded on DAD are insignificantly shifted relative to fluorescence detector because of the series connection of both detectors.
Obtained LOD values for biogenic amines are lower than that given by Adão and Glória (2005) or Vasconcelos et al. (2007), who have been using similar detector (FL). Values obtained by them were subsequently 30–50 ng/mL and 1,500 ng/mL. For methylxanthines, LOD values obtained in our research were comparable to that specified in the literature (Tzanavarasa et al. 2010).
Food which contains both biogenic amines and methylxanthines (chocolate with 90 % cocoa and cocoa) and foodstuff containing levodopa (broad beans and beans) were examined. Applied method of food sample preparation is suitable for all of examined foodstuff and allows one good separation of all analytes from the matrix. Within-day and between-day precision and accuracy of the applied method for three different additions of levodopa, biogenic amines, and methylxanthines from two different matrixes have been examined. First matrix was cocoa, which is similar to chocolate and the second one was broad bean matrix, which is similar to the beans. The applied sample preparation method allowed one to achieve recoveries from 72.0 to 99.8 % for the biogenic amines in both cocoa and broad beans matrix, whereas from 80 to 99 % for the methylxanthines in coca matrix. The obtained results concerning cocoa matrix are presented in the first part of Table 2, and in second part of this, Table 2 obtained results concerning broad beans matrix are presented. There were no examinations of recoveries of methylxanthines from broad bean matrix because those analytes do not occur in real samples. For the same reason, research of recovery of levodopa has only been performed for broad bean matrix.
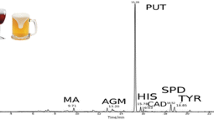
In the examined chocolate and cocoa samples, the following biogenic amines and methylxanthines were determined: norepinephrine (R t = 2.05 min), normetanephrine (R t = 4.25 min), dopamine (R t = 5.61 min), tyramine (R t = 12.10 min), serotonin (R t = 15.72 min), and theophylline (R t = 16.28 min, R t = 16.69 min), theobromine (R t = 18.53 min, R t = 18.85 min), and caffeine (R t = 26.52 min, R t = 26.89 min). In the case of methylxanthines, analysis with the use of both detectors at the same time is possible. The first of retention times come from chromatograms recorded with DAD and the second from fluorescence detector. In the examined broad bean samples, the following compounds were determined: levodopa (R t = 3.24 min), normetanephrine (R t = 4.02 min), and tyramine (R t = 10.05 min). Exemplary chromatogram of a cocoa sample from fluorescence detector is shown in Fig. 2a. Chromatogram of the same cocoa sample, but recorded on DAD, is shown in Fig. 2b. Unidentified peaks at chromatogram come from the cocoa matrix. Exemplary chromatogram of broad bean is shown in Fig. 3. Because there is no methylxanthines in the broad bean, there were no peaks from methylxanthines on the chromatogram from DAD. The differences between retention times of the analytes in model samples and in real food sample are results of a matrix effect. The presence of particular analytes was confirmed by the method of adding standards as well as basing on the UV spectra within the range of 200–600 nm. The concentration of analytes in food samples are presented in Table 3.
A higher methylxanthines concentration value in cacao than in chocolate results from fact that cacao is the only source of those compounds in chocolate. Other components of the final product lower the methylxanthines concentration value in chocolate. Concentration of dopamine and serotonin in chocolate fits in the ranges described by Pastore et al. (2005), where 96 μg of dopamine and 61 μg of serotonin in 1 g of chocolate had been determined.
Procedures described in literature allowed one to extract several compounds from one group and to apply particular procedure to one type of foodstuff only. This resulted in longer time of analytical process in the case of an analysis of various substances or foodstuff because it required different sample preparation procedure and different chromatographic methods for each food stuff (Matissek 1997).
Conclusion
Developed chromatographic system allows one to determine concentration of levodopa, norepinephrine, dopamine, normetanephrine, tyramine, serotonin, and theobromine, theophylline, and caffeine in foodstuffs. The procedure of food sample preparation is quick and easy to perform. Determined validation parameters—LOD, LOQ, linearity range, and recoveries of real samples for developed procedures—show that they can be used in routine food analysis.
Until now, no chromatographic system that allows simultaneous determination of dopamine, serotonin, and caffeine and its metabolites has been developed. In this work, it is shown that simultaneous determination of those analytes is possible not only by using the serial system of detectors DAD-FL but also with the use of fluorescence detector only, which has not been used for the determination of methylxanthines.
Thanks to those reasons the developed method could be applied for food analysis not only for monitoring concentration of some biogenic amines (e.g., tyramine) but also for diet development for people with some kind of illness. It should be emphasized that until now there have been no publicized records of a chromatographic system able to simultaneously determine all of the examined compounds in food samples.
References
Adão RC, Glória MBA (2005) Bioactive amines and carbohydrate changes during ripening of ‘Prata’ banana (Musa acuminata x M. balbisiana). Food Chem 90:705–711
Baranowska I, Płonka J (2008) Determination of levodopa and biogenic amines in urine samples using high-performance liquid chromatography. J Chromatogr Sci 46:30–34
Baranowska I, Zydroń M (2002) Liquid chromatography in the analysis of neurotransmitters and alkaloids. J Chromatogr Sci 40:224–228
Baranowska I, Płonka J, Baranowski J (2006) HPLC in analysis of methylxanthines and selected drugs in urine samples. Chem Anal (Warsaw) 51:751–760
Biagio Mercuri N, Bernardi G (2005) The ‘magic’ of l-dopa: why is it the gold standard Parkinson’s disease therapy? Trends Pharmacol Sci 26:341–344
Bruinsma K, Tarner DL (1999) Chocolate: food or drug? J Am Diet Assoc 99:1249–1256
Calbiani F, Careri M, Elviri L, Mangia A, Pistara L, Zagnoni I (2005) Rapid assay for analyzing biogenic amines in cheese: matrix solid-phase dispersion followed by liquid chromatography-electrospray-tandem mass spectrometry. J Agric Food Chem 53:3779–3783
Caudle AG, Gu Y, Bell LN (2001) Improved analysis of theobromine and caffeine in chocolate food products formulated with cocoa powder. Food Res Int 34:599–603
Chen X, Zhang J, Zhai H, Chen X, Hu Z (2005) Determination of levodopa by capillary zone electrophoresis using an acidic phosphate buffer and its application in the analysis of beans. Food Chem 92:381–386
Chiacchierini E, Restuccia D, Vinci G (2006) Evaluation of two different extraction methods for chromatographic determination of bioactive amines in tomato products. Talanta 69:548–555
Ciriloa MPG, Coelhoa AFS, Araújo CM, Gonçalves FRB, Nogueira FD, Glória MBA (2003) Profile and levels of bioactive amines in green and roasted coffee. Food Chem 82:397–402
Dadáková E, Křížek M, Pelikánová T (2009) Determination of biogenic amines in foods using ultra-performance liquid chromatography (UPLC). Food Chem 116:365–370
Favaro G, Pastore P, Saccani G, Cavalli S (2007) Determination of biogenic amines in fresh and processed meat by ion chromatography and integrated pulsed amperometric detection on Au electrode. Food Chem 105:1652–1658
Halász A, Baráth Á (2002) Toxicity of biogenic amines—the present knowledge. COST 917:131–141
Hernández-Orte P, Lapeña AC, Peña-Gallego A et al (2008) Biogenic amine determination in wine fermented in oak barrels: factors affecting formation. Food Res Int 41:697–706
Hicks MB, Hsieh Y-HP, Bell LN (1996) Tea preparation and its influence on methylxanthine concentration. Food Res Int 29:325–330
Higashimoto M, Akada Y, Sato M, Kinouchi T, Kuwahara T, Ohnishi Y (2000) Inhibitory effects of tea extracts on the mutagenicity of 1-methyl-1,2,3,4-tetrahydro-b-carboline-3-carboxylic acid on treatment with nitrite in the presence of ethanol. Food Chem Toxicol 38:7–13
Hlabangana L, Hernández-Cassou S, Saurina J (2006) Determination of biogenic amines in wines by ion-pair liquid chromatography and post-column derivatization with 1,2-naphthoquinone-4-sulphonate. J Chromatogr A 1130:130–136
Isolabella S, Cogoi L, López P, Anesini C, Ferraro G, Filip R (2010) Study of the bioactive compounds variation during yerba mate (Ilex paraguariensis) processing. Food Chem 122:695–699
Latorre-Moratalla ML, Bosch-Fustéa J, Lavizzaria T, Bover-Cidb S, Veciana-Noguésa MT, Vidal-Caroua MC (2009) Validation of an ultra high pressure liquid chromatographic method for the determination of biologically active amines in food. J Chromatogr A 1216:7715–7720
Lavizzari T, Veciana-Nogues MT, Bover-Cid S, Marine-Font A, Vidal-Carou MC (2006) Improved method for the determination of biogenic amines and polyamines in vegetable products by ion-pair high-performance liquid chromatography. J Chromatogr A 1129:67–72
Matissek R (1997) Evaluation of xanthine derivatives in chocolate—nutritional and chemical aspects. Z Lebensmitteluntersuchung Forsch A 205:175–184
Mayer HK, Fiechter G, Fischer E (2010) A new ultra-pressure liquid chromatography method for the determination of biogenic amines in cheese. J Chromatogr A 1217:3251–3257
McCabe-Sellers BJ, Staggs CG, Bogle ML (2006) Tyramine in foods and monoamine oxidase inhibitor drugs: a crossroad where medicine, nutrition, pharmacy, and food industry converge. J Food Compos Anal 19:S58–S65
Melzig MF, Putscher I, Henklein P, Haber H (2000) In vitro pharmacological activity of the tetrahydroisoquinoline salsolinol present in products from Theobroma cacao L. like cocoa and chocolate. J Ethnopharmacol 73:153–159
Nishi Y, Sasaki K, Miyatake T (2010) Biogenic amines, caffeine and tonic immobility in Tribolium castaneum. J Insect Physiol 56:622–628
Oliveira SD, Franca AS, Glória MBA, Borges MLA (2005) The effect of roasting on the presence of bioactive amines in coffees of different qualities. Food Chem 90:287–291
Pastore P, Favaro G, Badocco D, Tapparo A, Cavalli S, Saccani G (2005) Determination of biogenic amines in chocolate by ion chromatographic separation and pulsed integrated amperometric detection with implemented waveform at Au disposable electrode. J Chromatogr A 1098:111–115
Roig A (2002) The role of biogenic amines as quality indicators. COST 917:171–174
Romero R, Gázquez D, Bagur MG, Sánchez-Viñas M (2000) Optimization of chromatographic parameters for the determination of biogenic amines in wines by reversed-phase high-performance liquid chromatography. J Chromatogr A 871:75–83
Shalaby AR (1996) Significance of biogenic amines to food safety and human health. Food Res Int 29:675–690
Siddhuraju P, Becker K (2001) Rapid reversed-phase high performance liquid chromatographic method for the quantification of l-Dopa (l-3,4-dihydroxyphenylalanine), non-methylated and methylated tetrahydroisoquinoline compounds from Mucuna beans. Food Chem 72:389–394
Tang F, Tao L, Luo X et al (2006) Determination of octopamine, synephrine and tyramine in citrus herbs by ionic liquid improved ‘green’ chromatography. J Chromatogr A 1125:182–188
Tipton KF (2002) Biogenic amines, diet and the properties of amine oxidases. COST 917:117–121
Tzanavarasa PD, Zacharisa CK, Themelisa DG (2010) Rapid determination of methylxanthines in real samples by high-performance liquid chromatography using the new FastGradient® narrow-bore monolithic column. Talanta 81:1494–1501
Vale S, Glória MBA (1998) Biogenic amines in Brazilian cheeses. Food Chem 63:343–348
Vasconcelos ALS, Franca AS, Glória MBA, Mendonça JCF (2007) A comparative study of chemical attributes and levels of amines in defective green and roasted coffee beans. Food Chem 101:26–32
Vinci G, Antonelli ML (2002) Biogenic amines: quality index of freshness in red and white meat. Food Control 13:519–524
Yashin YI, Yashin AY (2004) Analysis of food products and beverages using high-performance liquid chromatography and ion chromatography with electrochemical detectors. J Anal Chem 59:1121–1127
Yigit M, Ersoy L (2003) Determination of tyramine in cheese by LC-UV. J Pharm Biomed Anal 31:1223–1228
Zydroń M, Baranowski J, Baranowska I (2004) Separation, pre-concentration and HPLC analysis of methylxanthines in urine samples. J Sep Sci 27:1166–1172
Zydroń M, Baranowski J, Białkowski J, Baranowska I (2005) HPLC-FL/ED in the analysis of biogenic amines and their metabolites in urine. Sep Sci Technol 40:3137–3148
Acknowledgments
This work was supported from science funds for scientific research for the years 2007–2008 (Project No. N N204 259434).
Conflict of Interest
Irena Baranowska has no conflict of interest. Joanna Płonka has no conflict of interest. This article does not contain any studies on human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Baranowska, I., Płonka, J. Simultaneous Determination of Biogenic Amines and Methylxanthines in Foodstuff—Sample Preparation with HPLC-DAD-FL Analysis. Food Anal. Methods 8, 963–972 (2015). https://doi.org/10.1007/s12161-014-9972-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-014-9972-x