Abstract
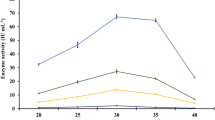
The mechanism underlying the production of fungal lignocellulolytic enzymes from lignocellulosic biomass remains unclear; therefore, it is imperative to investigate the interactions of microorganisms, culture components, and enzyme production. In this research, four native fungi isolated from agave bagasse: Aspergillus fumigatus (BC-1IB and BDJ-1S), Neurospora sitophila (BDJ-1I), and Rhizopus oryzae (BC-3IDA), were used to evaluate their interaction with lignocellulosic biomass as carbon source (agave or sugarcane bagasse) and pH (6.4 and 7.9) during the production of enzymatic extracts by solid-state fermentation with a packed-bed bioreactor. The evaluated enzymatic activities were pectinolytic, cellulolytic, and xylanolytic. The results demonstrate that the interaction between fungal strain, biomass, and pH during solid-state fermentation has a relevant effect on the final catalytic properties of the resulting enzymatic extract. The interaction between BDJ-1I and agave bagasse results in a pectinase extract that preserves above 99% of its activity at 70 °C for 5 h. Notably, the interaction between N. sitophila BDJ-1I with agave bagasse at pH 6.4 results in extracts with higher pectinase (96.8 IU/gdw), cellulase (39.3 IU/gdw), and xylanase (26.6 IU/gdw) activities suitable for hydrolyzing agro-industrial residues like wheat straw (30.2%), sugarcane (17.4%), and agave bagasse (28.4%). These findings highlight reusing agave and sugarcane bagasse residues for biotransformation into lignocellulosic enzyme cocktails with naturally present fungi strains toward the sustainable zero-waste management of agave and sugarcane bagasse biomass.
Graphical Abstract







Similar content being viewed by others
References
Perea-Moreno MA, Samerón-Manzano E, Perea-Moreno AJ (2019) Biomass as renewable energy: worldwide research trends. Sustain. 11. https://doi.org/10.3390/su11030863
Sandoval-Nuñez D, Arellano-Plaza M, Gschaedler A, Arrizon J, Amaya-Delgado L (2018) A comparative study of lignocellulosic ethanol productivities by Kluyveromyces marxianus and Saccharomyces cerevisiae. Clean Techn Environ Policy 20:1491–1499. https://doi.org/10.1007/s10098-017-1470-6
Amaya-Delgado L, Flores-Cosío G, Sandoval-Nuñez D, Arellano-Plaza M, Arrizon J, Gschaedler A (2018) Comparative of lignocellulosic ethanol production by Kluyveromyces marxianus and Saccharomyces cerevisiae, in: Special Topics in Renewable Energy Systems. IntechOpen. https://doi.org/10.5772/intechopen.78685
Carrillo-Nieves D, Saldarriaga-Hernandez S, Gutiérrez-Soto G, Rostro-Alanis M, Hernández-Luna C, Alvarez AJ, Iqbal HMN, Parra-Saldívar R (2020) Biotransformation of agro-industrial waste to produce lignocellulolytic enzymes and bioethanol with a zero waste. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-020-00738-6
Gonzalez-Rios JA, Valle-Pérez AU, Amaya-Delgado L, Sanchez A (2021) A quick fed-batch saccharification strategy of wheat straw at high solid loadings improving lignocellulosic ethanol productivity. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-01580-0
Saldarriaga-Hernández S, Velasco-Ayala C, Leal-Isla Flores P, de Jesús Rostro-Alanis M, Parra-Saldivar R, Iqbal HMN, Carrillo-Nieves D (2020) Biotransformation of lignocellulosic biomass into industrially relevant products with the aid of fungi-derived lignocellulolytic enzymes. Int J Biol Macromol 161:1099–1116. https://doi.org/10.1016/j.ijbiomac.2020.06.047
Valle-Pérez AU, Flores-Cosío G, Amaya-Delgado L (2021) Bioconversion of agave bagasse to produce cellulases and xylanases by Penicillium citrinum and Aspergillus fumigatus in solid-state fermentation. Waste Biomass Valorization. https://doi.org/10.1007/s12649-021-01397-y
Lagunes-Reyes M, Sánchez J, Andrade-Gallegos R, Gutiérrez-Hernández R, Camacho-Morales R (2023) Biodegradation of agave Comiteco bagasse by Pleurotus spp: a source of cellulases useful in hydrolytic treatment to produce reducing sugars. 3 Biotech 13:356. https://doi.org/10.1007/s13205-023-03783-w
Silva-Mendoza J, Gómez-Treviño A, López-Chuken U, Blanco-Gámez EA, Chávez-Guerrero L, Cantú-Cárdenas ME (2020) Agave leaves as a substrate for the production of cellulases by Penicillium sp. and the obtainment of reducing sugars. J Chem 2020:1–7. https://doi.org/10.1155/2020/6092165
Sadh PK, Duhan S, Duhan JS (2018) Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour Bioprocess 5:1–15. https://doi.org/10.1186/s40643-017-0187-z
Sarsaiya S, Awasthi SK, Awasthi MK, Awasthi AK, Mishra S, Chen J (2018) The dynamic of cellulase activity of fungi inhabiting organic municipal solid waste. Bioresour Technol 251:411–415. https://doi.org/10.1016/j.biortech.2017.12.011
de Oliveira Rodrigues P, dos Santos BV, Costa L, Henrique MA, Pasquini D, Baffi MA (2017) Xylanase and β-glucosidase production by Aspergillus fumigatus using commercial and lignocellulosic substrates submitted to chemical pre-treatments. Ind Crop Prod 95:453–459. https://doi.org/10.1016/j.indcrop.2016.10.055
Kumar A, Rani R, Pandey A (2017) Chapter 2 - Production, purification, and application of microbial enzymes. In: Biotechnology of Microbial Enzymes, pp 13–41. https://doi.org/10.1016/B978-0-12-803725-6.00002-9
Ramoni J, Marchetti-Deschmann M, Seidl-Seiboth V, Seiboth B (2017) Trichoderma reesei xylanase 5 is defective in the reference strain QM6a but functional alleles are present in other wild-type strains. Appl Microbiol Biotechnol 101:4139–4149. https://doi.org/10.1007/s00253-017-8161-4
Ravindran R, Hassan SS, Williams GA, Jaiswal AK (2018) A review on bioconversion of agro-industrial wastes to industrially important enzymes. Bioengineering 5:1–20. https://doi.org/10.3390/bioengineering5040093
Dhanya BS, Mishra A, Chandel AK, Verma ML (2020) Development of sustainable approaches for converting the organic waste to bioenergy. Sci Total Environ 723:138109. https://doi.org/10.1016/j.scitotenv.2020.138109
Jayasekara S, Ratnayake R (2019) Microbial cellulases: an overview and applications, in: Cellulose. IntechOpen. https://doi.org/10.5772/intechopen.84531
Singh A, Rodríguez Jasso RM, Gonzalez-Gloria KD, Rosales M, Belmares Cerda R, Aguilar CN, Singhania RR, Ruiz HA (2019) The enzyme biorefinery platform for advanced biofuels production. Bioresour Technol Rep 7:100257. https://doi.org/10.1016/j.biteb.2019.100257
Tarafdar A, Sirohi R, Gaur VK, Kumar S, Sharma P, Varjani S, Pandey HO, Sindhu R, Madhavan A, Rajasekharan R, Sim SJ (2021) Engineering interventions in enzyme production: lab to industrial scale. Bioresour Technol 326:124771. https://doi.org/10.1016/j.biortech.2021.124771
Peña-Maravilla M, Calixto-Romo MA, Guillen-Navarro K, Sanchez J, Amaya-Delgado L (2017) Cellulases and xylanases production by Penicillium citrinum CGETCR using coffee pulp in solid state fermentation. Rev Mex Ing Quim 16:757–769
Shinkawa S, Mitsuzawa S (2020) Feasibility study of on-site solid-state enzyme production by Aspergillus oryzae. Biotechnol Biofuels 13:31. https://doi.org/10.1186/s13068-020-1669-3
Infanzón-Rodríguez MI, Ragazzo-Sánchez JA, del Moral S, Calderón-Santoyo M, Aguilar-Uscanga MG (2020) Production and characterization of an enzyme extract with cellulase activity produced by an indigenous strain of Fusarium verticillioides ITV03 using sweet sorghum bagasse. Biotechnol Lett:0123456789. https://doi.org/10.1007/s10529-020-02940-y
Siqueira JG, Rodrigues C, de Souza Vandenberghe LP, Woiciechowski AL, Soccol CR (2020) Current advances in on-site cellulase production and application on lignocellulosic biomass conversion to biofuels: a review. Biomass Bioenergy 132:105419. https://doi.org/10.1016/j.biombioe.2019.105419
Bala A, Singh B (2019) Cellulolytic and xylanolytic enzymes of thermophiles for the production of renewable biofuels. Renew Energy 136:1231–1244. https://doi.org/10.1016/j.renene.2018.09.100
GVR. (2023). Enzymes Market Size, Share and Trends [WWW Document]. URL https://www.grandviewresearch.com/industry-analysis/enzymes-industry
Ozcirak Ergun S, Ozturk Urek R (2017) Production of ligninolytic enzymes by solid state fermentation using Pleurotus ostreatus. Ann Agrar Sci 15:273–277. https://doi.org/10.1016/j.aasci.2017.04.003
Mtibaà R, de Eugenio L, Ghariani B, Louati I, Belbahri L, Nasri M, Mechichi T (2017) A halotolerant laccase from Chaetomium strain isolated from desert soil and its ability for dye decolourization. 3 Biotech 7:329. https://doi.org/10.1007/s13205-017-0973-5
Rajagopalan G, Yew KW, He J, Yang KL (2013) Production, purification, and characterization of a xylooligosaccharides-forming xylanase from high-butanol-producing strain Clostridium sp. BOH3. Bioenergy Res 6:448–457. https://doi.org/10.1007/s12155-012-9259-2
Sousa D, Salgado J, Cambra-Lopez M, Dias A, Belo I (2021) Degradation of lignocellulosic matrix of oilseed cakes by solid-state fermentation: fungi screening for enzymes production and antioxidants release. J Sci Food Agric. https://doi.org/10.1002/jsfa.11490
Durand A (2003) Bioreactor designs for solid state fermentation. Biochem Eng J 13:113–125. https://doi.org/10.1016/S1369-703X(02)00124-9
McCleary BV, McGeough PA (2015) Comparison of polysaccharide substrates and reducing sugar methods for the measurement of endo-1,4-β-Xylanase. Appl Biochem Biotechnol 177:1152–1163. https://doi.org/10.1007/s12010-015-1803-z
Amit K, Nakachew M, Yilkal B, Mukesh YJRJCE (2018) A review of factors affecting enzymatic hydrolysis of pretreated lignocellulosic biomass. Res J Chem Environ 22(7):62–67
Cerda A, Mejías L, Gea T, Sánchez A (2017) Cellulase and xylanase production at pilot scale by solid-state fermentation from coffee husk using specialized consortia: the consistency of the process and the microbial communities involved. Bioresour Technol 243:1059–1068. https://doi.org/10.1016/j.biortech.2017.07.076
dos Santos BV, Rodrigues PO, Albuquerque CJB, Pasquini D, Baffi MA (2019) Use of an (hemi) cellulolytic enzymatic extract produced by Aspergilli species Consortium in the saccharification of biomass sorghum. Appl Biochem Biotechnol 189:37–48. https://doi.org/10.1007/s12010-019-02991-6
Kulkarni N, Vaidya T, Rathi G (2018) Optimization of cellulase production by Aspergillus species under solid state fermentation. Pharm Innov 7:193–196. https://doi.org/10.22271/tpi
Ortiz GE, Ponce-Mora MC, Noseda DG, Cazabat G, Saravalli C, López MC, Gil GP, Blasco M, Albertó EO (2017) Pectinase production by Aspergillus giganteus in solid-state fermentation: optimization, scale-up, biochemical characterization and its application in olive-oil extraction. J Ind Microbiol Biotechnol 44:197–211. https://doi.org/10.1007/s10295-016-1873-0
Oumer OJ, Abate D (2018) Comparative studies of pectinase production by Bacillus subtilis strain Btk 27 in submerged and solid-state fermentations. Biomed Res Int. https://doi.org/10.1155/2018/1514795
Wang J, Chio C, Chen X, Su E, Cao F, Jin Y, Qin W (2019) Efficient saccharification of agave biomass using Aspergillus niger produced low-cost enzyme cocktail with hyperactive pectinase activity. Bioresour Technol 272:26–33. https://doi.org/10.1016/j.biortech.2018.09.069
Li Y, Peng X, Chen H (2013) Comparative characterization of proteins secreted by Neurospora sitophila in solid-state and submerged fermentation. J Biosci Bioeng 116:493–498. https://doi.org/10.1016/j.jbiosc.2013.04.001
Lenartovicz V, Marques De Souza CG, Moreira FG, Peralta RM (2002) Temperature effect in the production of multiple xylanases by Aspergillus fumigatus. J Basic Microbiol 42:388–395. https://doi.org/10.1002/1521-4028(200212)42:6<388::AID-JOBM388>3.0.CO;2-H
Singh R, Kumar M, Mittal A, Mehta PK (2016) Microbial enzymes: industrial progress in 21st century. 3 Biotech 6:1–15. https://doi.org/10.1007/s13205-016-0485-8
Walia A, Guleria S, Mehta P, Chauhan A, Parkash J (2017) Microbial xylanases and their industrial application in pulp and paper biobleaching: a review. 3 Biotech 7:11. https://doi.org/10.1007/s13205-016-0584-6
Perez-Pimienta JA, Lopez-Ortega MG, Chavez-Carvayar JA, Varanasi P, Stavila V, Cheng G, Singh S, Simmons BA (2015) Characterization of agave bagasse as a function of ionic liquid pretreatment. Biomass Bioenergy 75:180–188. https://doi.org/10.1016/j.biombioe.2015.02.026
Bassim Atta M, Ruiz-Larrera F (2021) Fungal pectinases in food technology. IntechOpen. https://doi.org/10.5772/intechopen.100910
Martin N, de Souza R, da Silva R, Gomes E (2004) Pectinase production by fungal strains in solid-state fermentation using agro-industrial bioproduct. Braz Arch Biol Technol 47(5). https://doi.org/10.1590/S1516-89132004000500018
Kumar V, Marín-Navarro J, Shukla P (2016) Thermostable microbial xylanases for pulp and paper industries: trends, applications and further perspectives. World J Microbiol Biotechnol 32:34. https://doi.org/10.1007/s11274-015-2005-0
Maleki M, Shahraki MF, Kavousi K, Ariaeenejad S, Hosseini Salekdeh G (2020) A novel thermostable cellulase cocktail enhances lignocellulosic bioconversion and biorefining in a broad range of pH. Int J Biol Macromol 154:349–360. https://doi.org/10.1016/j.ijbiomac.2020.03.100
Patel AK, Singhania RR, Sim SJ, Pandey A (2019) Thermostable cellulases: current status and perspectives. Bioresour Technol 279:385–392. https://doi.org/10.1016/j.biortech.2019.01.049
Pavón-Orozco P, Santiago-Hernández A, Rosengren A, Hidalgo-Lara ME, Stålbrand H (2012) The family II carbohydrate-binding module of xylanase CflXyn11A from Cellulomonas flavigena increases the synergy with cellulase TrCel7B from Trichoderma reesei during the hydrolysis of sugar cane bagasse. Bioresour Technol 104:622–630. https://doi.org/10.1016/j.biortech.2011.11.068
Lamounier KFR, Rodrigues PO, Pasquini D, Baffi MA (2018) Saccharification of sugarcane bagasse using an enzymatic extract produced by Aspergillus fumigatus. J Renew Mater 6:169–175. https://doi.org/10.7569/JRM.2017.634151
Acknowledgements
A.U. Valle-Pérez would like to thank the support from Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco (CIATEJ). We would also like to thank Montserrat del Socorro Valle Pérez for digital illustration of the graphical abstract.
Funding
This research was supported by the Energy Sustainability Fund 245750 (CONAHCYT-SENER).
Author information
Authors and Affiliations
Contributions
Conceptualization: LA-D; data curation, LA-D, AV-P, JHG-A; formal analysis: LA-D, GF-C, JHG-A; funding acquisition: LA-D; investigation: LA-D, JHG-A; methodology: JHG-A, AV-P; project administration: LA-D; software: AV-P; supervision and validation: LA-D; writing—original draft: AV-P; writing—review and editing: LA-D, AV-P, GF-C.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1095 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Valle-Pérez, A.U., Gómez-Angulo, J.H., Flores-Cosío, G. et al. Interaction of Fungal Strains, Biomass, and pH to Produce Lignocellulosic Enzymes in Solid-State Fermentation for Sustainable Biotransformation of Sugarcane and Agave Bagasse. Bioenerg. Res. 17, 1015–1028 (2024). https://doi.org/10.1007/s12155-023-10695-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-023-10695-3