Abstract
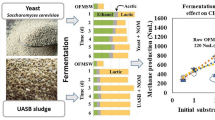
In the present study, the OFMSW was utilized for ethanol and methane production in a two-stage process. First, the OFMSW was fermented by adjusting different levels of total solids (10%, 20%, and 29%) and a yeast dose (6, 12, and 24 g/kgOFMSW) for bioaugmentation with Saccharomyces cerevisiae. A control treatment without bioaugmentation was used for benchmarking. In the self-fermentation of the OFMSW with its indigenous microbiota, lactic acid was mainly produced, followed by ethanol and acetic acid, where a maximum ethanol production of 113 ± 2 gCOD/kgTS, corresponding to 10.8 ± 0.4 g/L, at 20% of total solids was observed. The results showed that in all treatments with bioaugmentation, regardless of the content of total solids, the main metabolites were ethanol, lactic acid, and acetic acid, which represented more than 95% of the total metabolites. The ethanol concentration was increased by increasing the total solids and the yeast dose. The highest ethanol production was 407 ± 3 gCOD/kgTS with the bioaugmentation of 24 g/kgOFMSW and 20% of total solids, whereas the maximum ethanol concentration of 43.4 ± 0.1 g/L was obtained with bioaugmentation of 12 g/kgOFMSW and 29%. The methane potential of the raw OFMSW was 329 ± 5 NL-CH4/kgVS, whereas the solid digestates resulting from the fermentation exhibited similar or even higher methane yields than raw OFMSW. A maximum methane potential of 464 ± 2 NL-CH4/kgVS from the solid digestate obtained after fermentation of the OFMSW with bioaugmentation of 6 g/kgOFMSW of yeast and 10% of total solids was achieved. Thus, large amounts of ethanol and methane were achieved without the application of pretreatment and/or enzymatic hydrolysis, which implies a low-cost alternative for the production of biofuels.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Ethanol and methane are renewable and sustainable energy sources that have a lower environmental impact than fossil fuels and both can be obtained through anaerobic digestion (AD) of organic matter [1]. There are two well-known AD processes to produce both biofuels from biomass: in a single-stage (SS-AD) or through two-stage (TS-AD) [2]. Nevertheless, it has been demonstrated that sequential production of value-added products and biofuels in a two-stage process improved the overall energy recovery of several substrates such as the organic fraction of municipal solid waste (OFMSW) [3, 4].
In recent years, the production of ethanol and methane from the OFMSW has received attention as a viable alternative to fossil fuels due to its high amounts of macro and micronutrients such as carbohydrates (30–69%), lipids (10–40%), and proteins (5–15%) [1, 5]. The high carbohydrate content reflects the OFMSW potential in ethanol production [6], since about 51% of soluble chemical oxygen demand (CODS) are water-soluble carbohydrates (WSC) [7] that are readily biodegradable by microorganisms during fermentation [8]. Maximum production of 176 gCOD/kgTS of ethanol (8.4 g/L) at 10% of total solids concentration (TS) has been registered during OFMSW fermentation using only the indigenous microbiota. Moreover, ethanol is always produced after lactic acid regardless of the variation of parameters such as temperature and TS [7]. More importantly, lactic acid production is inversely proportional to the TS, whereas, in the case of ethanol, an increase in the TS is barely reduced in the production of this metabolite in comparison with the lactic acid decrease. These results indicate that to enhance ethanol production the OFMSW bioaugmentation with a strain that rapidly uptakes WSC is necessary and an increase in TS could favor alcohol formation [7].
Bioaugmentation involves the addition of exogenous microorganisms into a specific substrate to improve the process’s performance. In particular, yeast is the most common type of fermentative microorganism used for ethanol production [6]. The best-known yeast used in the OFMSW fermentation is Saccharomyces cerevisiae because of its wide range of pH tolerance [9], high tolerance to inhibitory compounds derived from biomass pretreatment [5], contamination, and side reactions [10]. Moreover, this yeast has a high affinity for glucose and it is also able to uptake other types of sugars such as fructose, sucrose, maltose, and maltotriose [11]. Bioaugmentation of OFMSW with this yeast enhanced the conversion of the available sugars after thermal, chemical [12], hydrothermal [13, 14], thermochemical pretreatments, and/or enzymatic hydrolysis to ethanol [5, 15, 16]. Frequently, the OFMSW is sterilized previous to yeast inoculation [6]. Nevertheless, it has been reported that non-sterilized fermentation is more advantageous compared to conventional sterilized fermentation [17]. Although previous studies demonstrated that the indigenous microbiota of the OFMSW is capable of establishing synergistic effects with an adapted microbial community [18, 19], no studies have been conducted about ethanol production when indigenous microbiota is enriched with S. cerevisiae.
The appropriate combination of operations units for ethanol and methane production can be regarded as a viable option to achieve a considerable bioenergy potential from biomass [20]. When AD is applied in a two-stage process, the production of metabolites during the first stage (hydrolysis, acidogenesis, and acetogenesis) can be controlled, allowing better performance of the second stage (methanogenesis). The ethanol produced after OFMSW fermentation is separated (through distillation or centrifugation) and the waste stream is anaerobically digested to produce biogas. Recovered solids are still an ideal substrate to generate methane in similar or even higher amounts than raw OFMSW [1, 5, 14, 21]. The controlled OFMSW fermentation to produce ethanol, by S. cerevisiae, and other metabolites such as lactic acid and volatile fatty acids (VFA) through indigenous microbiota [7], improve subsequent methanogenesis because metabolites such as acetic acid and ethanol are preferred substrates during methanization [22].
This research hypothesizes that ethanol production from the OFMSW can be enhanced by bioaugmentation of the substrate with microorganisms capable of the rapid uptake of WSC. Furthermore, an increase in the concentration of total solids would reduce lactic acid formation and favor ethanolic fermentation. The solid digestate recovered from the ethanolic fermentation would be able to produce more methane than the biochemical methane potential (BMP) of the raw OFMSW. Therefore, the study’s main objective was to determine the ethanol production from OFMSW fermentation under different total solid concentrations and S. cerevisiae doses, as well as the BMP tests from solid digestates, recovered from the selective fermentation to ethanol after the metabolite separation.
Materials and Methods
Feedstock
The OFMSW samples were collected at the Coyoacan transfer station in Mexico City where the sampling was made according to the Standard Test Method for Determination of the Composition of Unprocessed Municipal Solid Waste [23]. Approximately 150 kg of OFMSW from 15 trucks were collected and mixed, and about 1 ton was thoroughly mixed using a skid-steer loader and shovels. Then, the quartering method was used twice to reduce the OFMSW to approximately 200 kg. Undesired materials, such as plastic bags, stones, and wood, were manually separated. To reduce the particle size, the OFMSW was mechanically grounded by using an industrial blender and an extruder for meat; the resulting OFMSW was stored in two-liter freezing bags and frozen at − 20 °C. Table 1 depicts the characteristics of the raw OFMSW.
Experimental Design and OFMSW Fermentations
A freeze-dried commercial yeast (95% of dry mass) was used to carry out the ethanolic fermentation of the raw OFMSW. S. cerevisiae biomass was hydrated in a sterile beaker with 100 mL of fresh water at 35 °C for 5 min, the cells were immediately transferred to 2-L sealable glass bottles that contained the corresponding OFMSW/water mixtures for each experimental condition.
The raw OFMSW was fermented by adjusting different content of total solids (10, 20, and 29%) and a dose of S. cerevisiae (6, 12, and 24 g/kgOFMSW). A treatment with OFMSW containing its indigenous microbiota and no addition of yeast was used as the control treatment for benchmarking later on. For the dry fermentation condition (29%), only a yeast dose of 12 g/kgOFMSW was tested to evaluate the fermentation performance of S. cerevisiae using only the natural moisture of the OFMSW. The fermentations were carried out in duplicate in 2-L batch bioreactors under mesophilic conditions (35 °C) for 72 h without pH control.
The bioreactors were sampled through a lateral sampling port to evaluate the metabolite production and the WSC uptake. The total mass extracted during sampling did not exceed 10% of the total content of the bioreactor so as not to alter the process stability. At the end of fermentations, the digestates (mix of supernatant and solid digestate) were centrifugated at 3,000 rpm for 10 min to analyze the fermentation products, whereas the solid digestates were subjected to a BMP test. Moreover, the raw OFMSW was centrifuged under the same conditions and the remaining raw solid residue was also subjected to a BMP test.
BMP of the OFMSW
The BMP was determined from the raw OFMSW and its respective raw solid residue, as well as from the solid digestates of fermented OFMSW in 500-mL glass bottles with a working volume of 400 mL. The anaerobic granular sludge used as inoculum was taken from a UASB digester operated at 35 °C of a wastewater treatment plant located in the State of Mexico, Mexico. Before the tests, the inoculum was centrifuged at 2500 rpm for 10 min to remove soluble substances, then it was mixed with tap water to the original volume and centrifuged again [8, 24]. In all the BMP tests, 8 g of volatile solids of inoculum, 10 g of raw OFMSW or solid digestate, and 1 mL of a micronutrient solution (FeCl3·4H2O, 2,000; CoCl2·6H2O, 2,000; MnCl2·4H2O, 500; CuCl2·H2O, 30; ZnCl2, 50; H3BO3, 50; NiCl2·6H2O, 50; (NH4)6Mo7O24·4H2O, 90; EDTA, 1,000 and HCl, 1 mg/L) were placed [25]. Then, 0.1 M phosphate buffer was supplied to each bottle until it completed the rest of the working volume. The pH was adjusted to 7.1 ± 0.1 at the beginning of the assay. Bottles were flushed with nitrogen to ensure anaerobic conditions and incubated at 35 °C in duplicate. Biogas production was quantified by using an Automatic Methane Potential Test System (AMPTS II). For endogenous methane production determination, blanks containing anaerobic sludge, buffer solution, and micronutrient solution were run. The overall methane production was calculated by subtracting the measure of blank productions in each sample. Methane generation was expressed as specific methane production (SMP), i.e., normalized liter (NL) per kg of volatile solids.
Biodegradability of OFMSW
In this study, the biodegradability of raw OFMSW and solid digestates was calculated using Buswell’s equation, as shown in Eq. (1):
where:
-
\({\upeta }_{{\text{CH}}_{4}}\) is the cumulative SMP (NL/kgVS)
-
\({\upeta }_{{\text{t-CH}}_{4}}\) is the OFMSW theoretical methane potential (NL/kgVS)
Modelling of methane production from OFMSW
The experimental data were modeled by using an adaptation of the modified Gompertz model, which considers a diauxic behavior of the cumulative biogas production curves [26]. Their analytical expression is reported in Eq. (2):
where:
-
y, is the cumulative SMP at time t (NL/kgVS)
-
Ai, is the SMP of the substrate (NL/kgVS)
-
µmi, is the SMP rate (NL/kgVS·day)
-
λi, is the lag time (day)
Analytical Methods
Total solids (TS), volatile solids (VS), chemical oxygen demand (COD), total Kjeldahl (TKN), and ammonia nitrogen (NH4-N) were determined according to Standard Methods [27]. Soluble COD and WSC were determined after filtering the sample through a 0.45 µm membrane. WSC were determined according to the phenol–sulfuric-acid method proposed by DuBois et al. [28]. The samples for metabolite determination were previously filtered by using 0.45 and 0.22 µm filters, and the injected volume was 1 µL. Lactic acid was determined by using the spectrophotometric method according to Castellón-Zelaya and González-Martínez [7]. Methanol, ethanol, and VFA (acetic, propionic, isobutyric, butyric, isovaleric, valeric, and hexanoic acids) were determined by using a gas chromatograph (HP 5890 GC System) equipped with a flame ionization detector (FID), Stabilwax column – DA, with hydrogen as a carrier at a flow rate of 2 mL/min. The injector and detector temperatures were the same, 220 °C. The oven temperature ramp was programmed from 40 to 220 °C at 10 °C/min.
The biogas composition (CO2 and CH4) was determined by using a gas chromatograph (SRI 9610c) equipped with a thermal conductivity detector, stainless steel column packed with silica gel (8600-PK1A), helium as carrier gas with a flow of 15.7 mL/min. The oven and detector temperatures were 50 and 150 °C, respectively. The volume of the sample injected was 1 mL [22].
Statistical Analysis
A two-way analysis of variance (ANOVA) with an a-posteriori (post hoc) Tukey test was performed to determine significant differences among treatments at a confidence level of 95% using the Data Analysis tool of the Microsoft Excel 365 Professional Plus 2019. Also, the Solver tool of Microsoft Excel 365 Professional Plus 2019 was used to find kinetics parameters from the modified Gompertz model (Ai, µmi, and λi).
Results and Discussion
The OFMSW Fermentations
Consumption of Water-Soluble Carbohydrates
At the beginning of the OFMSW fermentations, 96% of the soluble COD was due to water-soluble carbohydrates (WSC), while the remaining percentage was caused by metabolites and ammonia nitrogen in the raw OFMSW. In the control treatment, the indigenous microbiota required 24 h to ferment 92% of the WSC when the TS was 10% (Fig. 1). When the TS increased to 20% and 29%, the indigenous microbiota required a longer time to consume the same quantity of WSC (48 h and longer). Moreover, in the fermentation at high-solid content (20% and 29% TS) the WSC increased gradually after 3 h.
Instead, when the OFMSW fermentations were bioaugmented with S. cerevisiae, WSC consumption was higher than in the control treatment (p ˂ 0.05), but no differences were observed among the three doses of yeast (p ˃ 0.05). For example, at the lowest TS of 10%, 77 ± 6% of the WSC were consumed in the first 6 h of fermentation, compared with 33% of the control treatment. This trend in the improvement of the WSC consumption was unchanged when the TS increased to 20% and 29% since all the bioaugmented treatments depleted the WSC before the control treatment. After 72 h of fermentation, WSC concentrations represented less than 5% concerning the initial concentration for all fermentation conditions.
Previous studies reported that WSC was reduced by 43% in 72 h during food waste fermentation at 6.5% of TS [29]. Nevertheless, during the food waste fermentation with indigenous microbiota at high-solid content, WSC accumulation was attributed to a higher rate of hydrolysis than acidogenesis [30]. This behavior might be because the high-solid loading increases the viscosity of the mixture and reduces the diffusion of WSC in the medium, which could limit the availability of dissolved substrate for the microorganisms and further hydrolysis of particulate organic matter is promoted 31.
When the OFMSW was bioaugmented with S. cerevisiae, WSC were consumed quickly in the first hours of fermentation. Wang et al. [32] reported a similar trend during the fermentation of kitchen waste for ethanol production. Moreover, the authors observed that more than 95% of reducing sugars were consumed in 60 h. In another study, Ntaikou et al. [16] also reported a reduction of 86% in total carbohydrates when a simultaneous saccharification and fermentation (SSF) process was applied to kitchen waste.
Distribution of Metabolites
Figure 1 depicts the metabolite production from the OFMSW fermentations only by the indigenous microbiota (control treatment) and bioaugmented with S. cerevisiae at different contents of TS and the yeast dose. In the self-fermentation of OFMSW by its indigenous microbiota, lactic acid was the predominant metabolite followed by ethanol and acetic acid, all together represented 95% of the total metabolites produced. The TS increase influenced the production of lactic acid only (p < 0.05), while the production of ethanol and acetic acid remained unchanged (p > 0.05). The TS increase from 10 to 20% doubled the lactic acid production from 227 ± 2 to 404 ± 3 gCOD/kgTS (p < 0.05), but the highest content of TS reduced its production to 344 ± 5 gCOD/kgTS. The TS also delayed the timing of the exponential phase: at the lowest TS, the exponential phase began after 6 h while for TS of 20% and 29%, the exponential phase was delayed 3 h.
The bioaugmentation with S. cerevisiae markedly changed the metabolite distribution since ethanol was the primary metabolite, followed by lactic and acetic acids. The yeast dose influenced the ethanolic fermentation depending on the content of TS (p < 0.05). At the lowest content of TS (10%), the increase in the yeast dose from 6 to 12 g/kgOFMSW improved the ethanol production by 46%, corresponding to 303 ± 11 gCOD/kgTS (p < 0.05), but the highest yeast dose did not affect it. In contrast, at the middle content of TS (20%), the highest yeast dose resulted in the highest ethanol production of 407 ± 11 gCOD/kgTS, and reducing the yeast dose decreased slightly the ethanol production (p ˃ 0.05). At the highest content of TS (29%), the ethanol production did not overcome the results obtained at a TS of 20%. Acetic acid production was not affected by the addition of yeast or TS increase (p ˃ 0.05) and the average production was 46 ± 10 gCOD/kgTS. Other metabolites such as methanol, propionic, isobutyric, and butyric acids also were detected at concentrations lower than 10 gCOD/kgTS. To elucidate the effect of yeast bioaugmentation on particulate organic matter fermentation, the remaining volatile solids in the solid digestates were quantified (Table 2). The resulting SV/ST ratios showed a higher removal of organic matter when the OFMSW was bioaugmented with S. cerevisiae than the self-fermentation with the indigenous microbiota.
The self-fermentation of the OFMSW resulted in a heterolactic fermentation where the primary products were lactic acid > ethanol > acetic acid regardless of the TS. These results agree with previous studies related to self-fermentation of OFMSW [7, 22, 33], food waste [34-37], and kitchen waste [38] in which lactic acid, ethanol, and acetic acid were the major products of the indigenous microbiota. Other studies also revealed that lactic and acetic acids were produced during self-fermentation of the OFMSW [39], kitchen waste [40], or food waste [41]. However, they did not report information on ethanol production. Furthermore, unlike ethanol and lactic acid, acetic acid accumulates over time. The trend of these data suggests that acetic acid was produced mainly by a process of acetogenesis and/or homoacetogenesis. Based on the reviewed literature, the indigenous microbiota of biowaste (OFMSW, food waste, kitchen waste, and household food waste) is mainly composed of lactic acid bacteria (LAB) capable of producing lactic acid, ethanol, and acetic acid (e.g., heterofermentative bacteria) [29, 36, 42].
In the present study, higher lactic acid production and concentration was achieved at the lowest TS than those reported by Tang et al. [30] and Castellón-Zelaya and González-Martínez [7]. The first authors registered values of 97 gCOD/kgTS (6.3 g/L) of lactic acid from food waste fermentation at 7% of TS, whereas the second one achieved a production of 192 gCOD/kgTS (17.8 g/L) from OFMSW at 10% of TS, using indigenous microbial community. When the TS increased, there was also an increase in lactic acid production. A similar trend was reported by Ahmad et al. [34] who tested the lactic acid production from food waste in dark fermentation with indigenous microbiota at different solids content. At TS of 20%, Castellón-Zelaya and González-Martínez [7] registered a production of 102 gCOD/kgTS (19 g/L), which was lower than that reported in the present study. Moreover, the previously cited authors reported a lactic acid production of 104 gCOD/kgTS (27 g/L) when the TS were 28%.
When S. cerevisiae was added to the OFMSW fermentation, ethanolic fermentation was predominant so ethanol was produced in higher amounts than lactic acid, whereas acetic acid production was almost unchanged. Ethanol production at 10% of TS was improved by increasing the yeast dose. Although the production rate was higher when OFMSW was bioaugmented with 24 g/kgOFMSW, ethanol production was similar to the lower dose. However, in the same condition, no effect was observed when TS was increased. High ethanol concentration at the beginning of the fermentation process can remarkably suppress the S. cerevisiae growth even when dissolved substrate (glucose) is available [43]. However, fermentations with a high solid content can limit the diffusion of ethanol in the medium, and the bactericidal effect of ethanol on microorganisms could be decreased. Furthermore, certain components of the complex substrate (as the particulate organic matter) may also improve the tolerance of yeast to various stresses [44].
By increasing the solids concentration from 10 to 20% and the yeast dose, ethanol production was improved. Huang et al. [45] reported similar results during the fermentation of pretreated corn stover with Baker’s yeast (S. cerevisiae). These authors achieved higher ethanol production as the TS content increased. Fermentation processes with a high TS content close to 20% can reduce bacterial contamination or even prevent it when values exceed 25%. While bacterial colonization can increase significantly at low TS (5, 10, and 15%) and reduce the WSC available to the yeast. Although lower ethanol production was achieved by increasing the TS to 29%, the production of lactic and acetic acids was similar to that of the previous conditions. These results indicate that the indigenous microbiota was able to produce these metabolites even when the yeast consumed the available WSC.
S. cerevisiae cannot produce enzymes to degrade particulate organic matter and depends on fermentable sugars availability [5]. On the contrary, LAB are capable of degrading polysaccharides (starch, cellulose, hemicellulose, etc.), proteins, and lipids into monosaccharides through the production of hydrolases [40]. Thus, the OFMSW can be hydrolyzed and dissolved into a liquid phase by enzymes secreted by LAB [36]. Because the yeast quickly depleted the WSC, LAB hydrolyzed the particulate organic matter and fermented it to lactic acid, ethanol, and acetic acid. According to data reported in Fig. 1, by increasing both the solids concentration from 10 to 20% and the yeast dose, ethanol production improves. This trend corresponded to the removal of volatile solids depicted in Table 2 since there was a higher consumption of organic matter when the TS content increased from 10 to 20%.
Indigenous microbiota of the OFMSW includes several taxa of lactic acid bacteria (LAB) such as Enterococcus [46], a genus that possesses xylanolytic activity [47]. In consequence, indigenous microbiota — particularly LAB — can perform simultaneously the hydrolysis and fermentation of particulate organic matter resulting in lactic acid production. Although the increase in the ratio of the fermentative members (S. cerevisiae) changed the fermentation products from lactic acid to ethanol, the production of lactic and acetic acids suggests that the LAB remained active and even improved fermentation. Previous studies also reported a better performance in ethanol production when fermentation was carried out without sterilization of media due to the indigenous microbiota of biowaste being capable of coexisting with another microbial community and promoting the hydrolysis process [18, 32].
The results presented in this study suggested a positive interaction— possibly synergy — between indigenous microbiota with S. cerevisiae since the quantity of soluble products after fermentation barely increased but the metabolite composition had a significant change. Previous studies report that a synergistic relationship can even be established by yeast with some genera of LAB and increase ethanol production due to the cross-feeding of acetaldehyde between both organisms. Acetaldehyde produced by the LAB acts as an alternative electron acceptor allowing the yeast cells to rebalance their NADH/NAD pools solely through ethanol production [48]. Villanueva-Galindo and Moreno-Andrade [49] observed that the concentration of microorganisms is a crucial parameter in the bioaugmentation strategy. However, further studies are required to determine which physicochemical parameters such as metabolite concentration, acidity, and temperature among others could promote the successful establishment of S. cerevisiae in the indigenous microbiota [50].
By adding S. cerevisiae without sterilizing the OFMSW, similar or even higher ethanol productions were achieved under wet fermentation conditions (≤ 10% TS) compared to the literature [1, 7, 14, 16, 21]. Furthermore, the increase of TS from 10 to 20% resulted in higher ethanol production and it is amongst the higher reported in the literature under semi-dry conditions (10% ˂ TS ≤ 20%) [5, 7, 32]. The highest ethanol concentration of 43.4 g/L was achieved at 29% of TS. This value achieved in the present research was higher than those reported in the literature when fermentation was carried out under dry conditions (> 20% TS) [7]. It should be highlighted that although there are several published literature focused on ethanol production from organic waste, no information is available on the production of metabolites when the OFMSW is fermented by its indigenous microbiota bioaugmented with S. cerevisiae at different dosages and TS content. Only Wang et al. [32] carried out the fermentation of unsterilized food waste, but only ethanol production was reported.
Most previous studies have used S. cerevisiae for ethanol production from the OFMSW, after pretreatment and/or enzymatic hydrolysis. However, endogenous enzymes secreted naturally by the indigenous microbiota could be an efficient way to solubilize the particulate organic matter without requiring commercial enzymes. The results of this study showed that in addition to producing ethanol by adding S. cerevisiae, it is possible to obtain other value-added products such as lactic and acetic acids by using the indigenous microbiota of the OFMSW without pretreatment and/or enzymatic hydrolysis.
Methane Production from OFMSW
Figure 2 depicts the BMP of the solid digestates recovered after the OFMSW fermentation under different conditions. A maximum SMP of 362 ± 11 NL-CH4/kgVS was achieved from solid digestate obtained after fermentation with indigenous microbiota at 10% of TS. At the same TS condition, when the OFMSW was bioaugmented with 6 g/kgOFMSW of yeast, a maximum SMP of 464 ± 2 NL-CH4/kgVS was achieved. In general, a reduction in the SMP was observed when the TS increased from 10 to 20% (p ˂ 0.05) during the OFMSW fermentation with the indigenous microbiota, but no significant differences were registered at the high-solid loading (29%; p > 0.05). When the OFMSW was bioaugmented with S. cerevisiae, the SMP of the remaining solid digestates was higher than methane production from the OFMSW fermented only with the indigenous microbiota (p ˂ 0.05). Moreover, significant differences in the SMP at different S. cerevisiae doses (p ˂ 0.05), and an inverse correlation between the methane productions and yeast dose were observed.
Experimental data and modeling of SMP of the raw OFMSW as well as their respective raw solid residue, and the solid digestates obtained during the fermentation stage. Control refers to solid digestate recovered after the self-fermentation of the OFMSW by its indigenous microbiota. Raw OFMSW was modeled with the modified Gompertz model in three phases
Data depicted in Table 2 showed that the VS removal was lower as the yeast dose increased, i.e., the availability of organic matter at the end of fermentation was higher when the OFMSW was bioaugmented with 24 g/kgOFMSW of yeast than in the lower doses. However, under these conditions, the lowest methane productions were reported. Then, SMP seems to be related to the availability of carbohydrates in the solid digestates. By increasing the TS and the yeast dose, ethanol production also increased, suggesting that both soluble as well as particulate carbohydrates were consumed during the fermentation stage by yeast and indigenous microbiota, respectively. Consequently, the amount of available carbohydrates was lower during the methanization stage, negatively affecting methane production. Nevertheless, according to Fig. 2, 86% of tests where the OFMSW was fermented previously exhibited a higher SMP than the raw OFMSW. While the methane production was higher in all fermentation conditions compared to the raw solid residue, where an increase in the SMP between 77 and 166% was attained.
Biodegradability
At standard temperature and pressure conditions (STP), the maximum amount of theoretical methane that can be obtained is 0.35 NL-CH4/gCOD. In this study, the CODT/VS ratio was 1.55 gCOD/gVS and a theoretical methane potential of 544 NL-CH4/kgVS was calculated for OFMSW of the Coyoacan transfer station. As reported in Fig. 2, excepting two tests carried out with 20% of TS (control and 24 g/kgOFMSW of yeast), the fermentation of raw OFMSW before methanization led to an increase in the biodegradability (ƞB) of the solid digestates both in the conditions where S. cerevisiae was added, as well as in cases where it was only fermented with the indigenous microbiota. The highest biodegradability percentages compared with the theoretical were 85% with a dose of 6 g/kgOFMSW of yeast at 10% of TS. Instead, the experiments where 24 g/kgOFMSW of yeast was added showed the lowest biodegradability values among all the experiments where OFMSW was bioaugmented with S. cerevisiae.
Castellón-Zelaya and González-Martínez [7] reported a SMP of 318 NL-CH4/kgVS for the raw OFMSW of the Coyoacan transfer station, this value was similar to that obtained in the present study. When the OFMSW was subjected to silage with indigenous microbiota (lactic fermentation), the SMP of digestates was low compared to the raw OFMSW. This reduction could be related to bacterial activity inhibition due to the transformation of lactic acid into propionic acid during AD [7]. Instead, when the OFMSW is subjected to fermentation with bioaugmentation of S. cerevisiae, the methane production from the solid digestates resulted in similar or even higher methane yields compared to the raw OFMSW. The SMP values achieved in the present study were amongst the higher reported in the literature even without pretreatment and/or enzymatic hydrolysis [1, 5, 14, 15, 21, 41].
The SMP improvement could be generated due to the solid digestates retained between 66 and 79% of moisture (ethanol, lactic and acetic acids) even after centrifugation to extract the supernatant that was subsequently transformed into methane. Thus, the ethanolic pre-fermentation of the OFMSW improved methane production by avoiding an acidification process and maintaining the stability of the anaerobic systems. This is mainly due to the carbon source being converted to ethanol. According to Jojoa‐Unigarro and González‐Martínez [22], both acetic acid and ethanol are preferred substrates for methane generation, whereas lactic acid has the lowest SMP. In particular, ethanol can improve direct interspecies electron transfer during AD, allowing higher energy recovery for the microorganisms than acetic acid or any other type of metabolite [2]. Moreover, during the acetogenesis stage, ethanol can be converted rapidly into acetic acid, allowing methanogens uptake acetate directly to produce methane.
Diauxic Behavior
All data depicted in Fig. 2 were fitted through the modified Gompertz model in two and three phases where all the curves exhibited a diauxic behavior. On the other hand, in the case of the raw OFMSW, three exponential phases were observed, suggesting that the bacterial growth behavior is triauxic.
In all cases, throughout the first 3 days of methanization, there was an exponential phase of methane production, later, for the solid digestate extracted from the fermentation with 10% and the raw solid residue, the stationary phase occurs between days 3 and 7 while a second exponential phase began from day 8. On the other hand, in the case of the solid digestates obtained from fermentation with 20%, the stationary phase was observed between days 3 and 8, i.e., one more day compared to the test with 10%, and from day 9 the second exponential phase of methane production was observed. In the tests with 29%, the stationary phase was visualized between days 3 and 9; later, from day 10, the second phase of higher methane production was observed. Instead, the raw OFMSW presented a second latency phase between days 14 and 19, and a third exponential phase from day 20 (Fig. 2), which was lower in comparison with the first and the second exponential phase.
Campuzano and González-Martínez [24] also reported a diauxic behavior in methane curves from OFMSW methanization. This trend is related to the mechanisms underlying enzymatic adaptation, where microorganisms consume an easily assimilable substrate A, and only when this is exhausted, they will synthesize enzymes to degrade a compound B and so then a C. During AD, a microbial community acts together to break down organic matter to produce biogas. An advantage of such consortia of microorganisms is their ability to synergistically break down various complex, recalcitrant, and inhibiting compounds (at low concentrations) to methane after some adaptation time (latency phase) [8, 24].
In this study, the biphasic trend suggests that, in the first exponential phase, the methanogens depleted rapidly the dissolved substrate (ethanol, AGV, lactic acid, etc.) and then produced methane. Nevertheless, when the dissolved substrate was exhausted, methanogens stopped methane production and the curve behavior exhibited a latency phase. This behavior could be associated with an acetogenesis process and subsequent methane production (second exponential phase) [22]. In the case of raw OFMSW methanization (Fig. 2), there was a third exponential phase that would indicate the presence of organic material that could be decomposed.
Modelling and Kinetic Parameters
All curves were modeled using an adaptation of the modified Gompertz model in two phases proposed by Gomes et al. [26] where A1, µm1, and λ1 are the kinetic parameters for the first phase, while A2, µm2, and λ2 were parameters of the second phase. In the case of raw OFMSW, due to the presence of three exponential phases, the mathematical model of Gompertz in three phases was applied. This modification to the model was mathematically based on the initial Gompertz model in two phases with three additional coefficients (A3, µm3, and λ3) and the coefficient of determination (R2) was even higher compared to the previous cases. Additional values for the raw OFMSW were A3 = 72.3 ± 3.5 NL/kgVS, µm3 = 12.2 ± 1.0 NL/kgVS·day, and λ3 = 18.5 ± 0.3 days. All kinetic parameters from this model are in Table 3.
As depicted in Table 3, the maximum methane production during the first exponential phase (A1) was lower than that obtained during the second exponential phase (A2). In general, between 32 and 40% of the methane accumulated in 28 days was produced during the first exponential phase. Moreover, only in the cases where yeast was added, A1 and A2 tended to reduce as the dose of S. cerevisiae increased. The sum of A1 and A2 was the cumulative SMP according to the modified Gompertz model in two phases, but in the case of raw OFMSW, it would be the sum of A1, A2, and A3. When analyzing the lambda parameter (λi), in the first latency phase (λ1), the values obtained were less than 0.3 days, suggesting that the inoculum went through a small adaptation phase and immediately consumed the dissolved substrate contained in the solid digestates with the consequent methane generation.
The methane production rate (µmi) decreased in each phase, i.e., µm1 > µm2. The results shown in Table 3 suggest that the trend in this parameter is indirectly related to the metabolism of the bacteria, i.e., µmi at the beginning of the BMP test is higher due to the rapid consumption of the dissolved substrate, which led to rapid methane production. Once the methanogens depleted the dissolved substrate, specialized bacteria secreted enzymes during the lag phase to hydrolyze the more complex substrate causing the rate to decrease in the second exponential phase because the dissolved substrate is only available as the complex substrate is hydrolyzed to compounds assimilable by methanogenic organisms [24]. In the case of raw OFMSW, the rate decreases even more, possibly because there is still a complex substrate that can be hydrolyzed.
Empirical kinetic models used in BMP tests vary by origin, including, but not limited to enzymatic or chemical or variable time kinetics, statistical distribution, and microbial growth models. However, the modified Gompertz model in two and even three phases are very useful to fit cumulative methane production curves which show a diauxic or triauxic behavior. Moreover, as shown in the present study, it is possible to increase the accuracy of the fit compared to the commonly modified Gompertz model.
Conclusions
Ethanol and methane production from OFMSW through a two-stage process was improved by increasing the TS content and adding S. cerevisiae. The highest ethanol yield was 407 ± 3.0 gCOD/kgTS with bioaugmentation of 24 g/kgOFMSW of yeast and 20% of TS, while the maximum ethanol concentration of 43.4 ± 0.1 g/L was obtained with lower bioaugmentation of 12 g/kgOFMSW of yeast and 29% of TS. The fermentation of raw OFMSW before methanization led to an increase in the methane yield, where 86% of the solid digestates produced methane (385 – 464 NL-CH4/kgVS) in higher amounts than the raw OFMSW.
Data Availability
All relevant data are included in the paper.
Code Availability
Not applicable.
References
Ebrahimian F, Karimi K, Kumar R (2020) Sustainable biofuels and bioplastic production from the organic fraction of municipal solid waste. Waste Manag 116:40–48. https://doi.org/10.1016/j.wasman.2020.07.049
Feng D, Guo X, Lin R et al (2021) How can ethanol enhance direct interspecies electron transfer in anaerobic digestion? Biotechnol Adv 52:107812. https://doi.org/10.1016/j.biotechadv.2021.107812
Calicioglu O, Brennan RA (2018) Sequential ethanol fermentation and anaerobic digestion increase bioenergy yields from duckweed. Bioresour Technol 257:344–348. https://doi.org/10.1016/j.biortech.2018.02.053
Wu C, Wang Q, Xiang J et al (2015) Enhanced Productions and Recoveries of Ethanol and Methane from Food Waste by a Three-Stage Process. Energy Fuels 29:6494–6500. https://doi.org/10.1021/acs.energyfuels.5b01507
Moreno AD, Magdalena JA, Oliva JM et al (2021) Sequential bioethanol and methane production from municipal solid waste: An integrated biorefinery strategy towards cost-effectiveness. Process Saf Environ Prot 146:424–431. https://doi.org/10.1016/j.psep.2020.09.022
Barampouti E, Mai S, Malamis D et al (2019) Liquid biofuels from the organic fraction of municipal solid waste: A review. Renew Sustain Energy Rev 110:298–314. https://doi.org/10.1016/j.rser.2019.04.005
Castellón-Zelaya MF, González-Martínez S (2021) Silage of the organic fraction of municipal solid waste to improve methane production. Water Sci Technol 83:2536–2548. https://doi.org/10.2166/wst.2021.148
Campuzano R, González-Martínez S (2015) Extraction of soluble substances from organic solid municipal waste to increase methane production. Bioresour Technol 178:247–253. https://doi.org/10.1016/j.biortech.2014.08.042
Dalena F, Senatore A, Iulianelli A, Di Paola L, Basile M, Basile A (2019) Ethanol from biomass: future an perspectives. In: Basile A, Iulianelli A, Dalena F, Veziroglu TN (eds) Ethanol: science and engineering. Elsevier, Amsterdam, pp 25–59. https://doi.org/10.1016/b978-0-12-811458-2.00002-x
Hong YS, Yoon HH (2011) Ethanol production from food residues. Biomass Bioenergy 35:3271–3275. https://doi.org/10.1016/j.biombioe.2011.04.030
Walker G, Stewart G (2016) Saccharomyces cerevisiae in the Production of Fermented Beverages. Beverages 2:30. https://doi.org/10.3390/beverages2040030
Cekmecelioglu D, Uncu ON (2013) Kinetic modeling of enzymatic hydrolysis of pretreated kitchen wastes for enhancing bioethanol production. Waste Manag 33:735–739. https://doi.org/10.1016/j.wasman.2012.08.003
Alamanou DG, Malamis D, Mamma D, Kekos D (2015) Bioethanol from dried household food waste applying non-isothermal simultaneous saccharification and fermentation at high substrate concentration. Waste Biomass Valor 6:353–361. https://doi.org/10.1007/s12649-015-9355-6
Mahmoodi P, Karimi K, Taherzadeh MJ (2018) Hydrothermal processing as pretreatment for efficient production of ethanol and biogas from municipal solid waste. Bioresour Technol 261:166–175. https://doi.org/10.1016/j.biortech.2018.03.115
Jarunglumlert T, Bampenrat A, Sukkathanyawat H, Prommuak C (2021) Enhanced Energy recovery from food waste by co-production of bioethanol and biomethane process. Fermentation 7:265. https://doi.org/10.3390/fermentation7040265
Ntaikou I, Menis N, Alexandropoulou M et al (2018) Valorization of kitchen biowaste for ethanol production via simultaneous saccharification and fermentation using co-cultures of the yeasts Saccharomyces cerevisiae and Pichia stipitis. Bioresour Technol 263:75–83. https://doi.org/10.1016/j.biortech.2018.04.109
Sakai K, Ezaki Y, Tongpim S, Kitpreechavanich V (2006) High-Temperature l-lactic acid fermentation of food waste under open condition and its FISH analysis of its micro flora. Agric Nat Resour 40:35–39
Dreschke G, Probst M, Walter A et al (2015) Lactic acid and methane: improved exploitation of biowaste potential. Bioresour Technol 176:47–55. https://doi.org/10.1016/j.biortech.2014.10.136
Zhang Z, Tsapekos P, Alvarado-Morales M, Angelidaki I (2021) Bio-augmentation to improve lactic acid production from source-sorted organic household waste. J Clean Prod 279:123714. https://doi.org/10.1016/j.jclepro.2020.123714
Cesaro A, Belgiorno V (2015) Combined biogas and bioethanol production: opportunities and challenges for industrial application. Energies 8:8121–8144. https://doi.org/10.3390/en8088121
Mahmoodi P, Karimi K, Taherzadeh MJ (2018) Efficient conversion of municipal solid waste to biofuel by simultaneous dilute-acid hydrolysis of starch and pretreatment of lignocelluloses. Energy Convers Manag 166:569–578. https://doi.org/10.1016/j.enconman.2018.04.067
Jojoa-Unigarro GD, Gonzalez-Martinez S (2022) Kinetic analysis of the methanization of the byproducts from OFMSW fermentation. J Chem Technol Biotechnol 9(6):1555–1566. https://doi.org/10.1002/jctb.6921
ASTM (2016) Standard test method for determination of the composition of unprocessed municipal solid waste. ASTM D5231. American Society for Testing Materials, West Conshohocken, PA. https://doi.org/10.1520/D5231-92R16
Campuzano R, González-Martínez S (2017) Influence of process parameters on the extraction of soluble substances from OFMSW and methane production. Waste Manag 62:61–68. https://doi.org/10.1016/j.wasman.2017.02.015
Verein Deutscher Ingenieure (2016) VDI 4630: Fermentation of organic materials. Characterisation of the substrate, sampling, collection of material data, fermentation tests. Beuth Verlag GmbH, Düsseldorf. http://refhub.elsevier.com/S0960-1481(19)30688-3/sref12
Gomes CS, Strangfeld M, Meyer M (2021) Diauxie Studies in biogas production from gelatin and adaptation of the modified gompertz model: two-phase Gompertz model. Appl Sci 11:1067. https://doi.org/10.3390/app11031067
APHA, AWWA, WEF (2017) Standard methods for the examination of water and wastewater, 23rd edn. American Public Health Association, Washington, DC
DuBois M, Gilles KA, Hamilton JK et al (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Tang J, Wang XC, Hu Y et al (2017) Effect of pH on lactic acid production from acidogenic fermentation of food waste with different types of inocula. Bioresour Technol 224:544–552. https://doi.org/10.1016/j.biortech.2016.11.111
Tang J, Wang X, Hu Y et al (2016) Lactic acid fermentation from food waste with indigenous microbiota: Effects of pH, temperature and high OLR. Waste Manag 52:278–285. https://doi.org/10.1016/j.wasman.2016.03.034
Nguyen TY, Cai CM, Kumar R, Wyman CE (2017) Overcoming factors limiting high-solids fermentation of lignocellulosic biomass to ethanol. Proc Natl Acad Sci USA 114:11673–11678. https://doi.org/10.1073/pnas.1704652114
Wang Q, Ma H, Xu W et al (2008) Ethanol production from kitchen garbage using response surface methodology. Biochem Eng J 39:604–610. https://doi.org/10.1016/j.bej.2007.12.018
Tsapekos P, Alvarado-Morales M, Baladi S et al (2020) Fermentative Production of lactic acid as a sustainable approach to valorize household bio-waste. Front Sustain 1:4. https://doi.org/10.3389/frsus.2020.00004
Ahmad A, Banat F, Taher H (2022) Enhanced lactic acid production from food waste in dark fermentation with indigenous microbiota. Biomass Conv Bioref 12:3425–3434. https://doi.org/10.1007/s13399-020-00801-2
Peinemann JC, Demichelis F, Fiore S, Pleissner D (2019) Techno-economic assessment of non-sterile batch and continuous production of lactic acid from food waste. Bioresour Technol 289:121631. https://doi.org/10.1016/j.biortech.2019.121631
Yang L, Chen L, Li H et al (2022) Lactic acid production from mesophilic and thermophilic fermentation of food waste at different pH. J Enviro Manag 304:114312. https://doi.org/10.1016/j.jenvman.2021.114312
Yousuf A, Bastidas-Oyanedel J-R, Schmidt JE (2018) Effect of total solid content and pretreatment on the production of lactic acid from mixed culture dark fermentation of food waste. Waste Manag 77:516–521. https://doi.org/10.1016/j.wasman.2018.04.035
Sakai K, Murata Y, Yamazumi H et al (2000) Selective proliferation of lactic acid bacteria and accumulation of lactic acid during open fermentation of kitchen refuse with intermittent pH adjustment. FSTR 6:140–145. https://doi.org/10.3136/fstr.6.140
Probst M, Walde J, Pümpel T et al (2015) Lactic acid fermentation within a cascading approach for biowaste treatment. Appl Microbiol Biotechnol 99:3029–3040. https://doi.org/10.1007/s00253-015-6414-7
Wang XM, Wang QH, Ren NQ, Wang XQ (2005) Lactic acid production from kitchen waste with a newly characterized strain of Lactobacillus plantarum. Chem Biochem Eng Q 19(4):383–389
García-Depraect O, Martínez-Mendoza LJ, Diaz I, Muñoz R (2022) Two-stage anaerobic digestion of food waste: Enhanced bioenergy production rate by steering lactate-type fermentation during hydrolysis-acidogenesis. Bioresour Technol 358:127358. https://doi.org/10.1016/j.biortech.2022.127358
Wilson LP, Sharvelle SE, De Long SK (2016) Enhanced anaerobic digestion performance via combined solids- and leachate-based hydrolysis reactor inoculation. Bioresour Technol 220:94–103. https://doi.org/10.1016/j.biortech.2016.08.024
Zhang Q, Wu D, Lin Y et al (2015) Substrate and Product Inhibition on Yeast Performance in Ethanol Fermentation. Energy Fuels 29:1019–1027. https://doi.org/10.1021/ef502349v
Moreno AD, González-Fernández C, Ballesteros M, Tomás-Pejó E (2019) Insoluble solids at high concentrations repress yeast’s response against stress and increase intracellular ROS levels. Sci Rep 9:12236. https://doi.org/10.1038/s41598-019-48733-w
Huang H, Guo X, Li D et al (2011) Identification of crucial yeast inhibitors in bio-ethanol and improvement of fermentation at high pH and high total solids. Bioresour Technol 102:7486–7493. https://doi.org/10.1016/j.biortech.2011.05.008
Villanueva-Galindo E, Vital-Jácome M, Moreno-Andrade I (2023) Dark fermentation for H2 production from food waste and novel strategies for its enhancement. Int J Hydrogen Energy 48:9957–9970. https://doi.org/10.1016/j.ijhydene.2022.11.339
Valdez-Vazquez I, Pérez-Rangel M, Tapia A et al (2015) Hydrogen and butanol production from native wheat straw by synthetic microbial consortia integrated by species of Enterococcus and Clostridium. Fuel 159:214–222. https://doi.org/10.1016/j.fuel.2015.06.052
de Oliveira S, Lino F, Bajic D, Vila JCC et al (2021) Complex yeast–bacteria interactions affect the yield of industrial ethanol fermentation. Nat Commun 12:1498. https://doi.org/10.1038/s41467-021-21844-7
Villanueva-Galindo E, Moreno-Andrade I (2021) Bioaugmentation on hydrogen production from food waste. Int J Hydrogen Energy 46:25985–25994. https://doi.org/10.1016/j.ijhydene.2020.11.092
Hao F, Tan Y, Lv X et al (2021) Microbial community succession and its environment driving factors during initial fermentation of Maotai-flavor Baijiu. Front Microbiol 12:669201. https://doi.org/10.3389/fmicb.2021.669201
Acknowledgements
This research was carried out at the Environmental Engineering Laboratory, Institute of Engineering, National University of Mexico. The graduate scholarship provided by CONACYT is acknowledged. The authors recognize the unconditional support in the laboratory of M.Sc. Francisco Rojo Callejas, Faculty of Chemistry, National University of Mexico.
Funding
This research was carried out thanks to the Support Program for Research and Technological Innovation Projects of the National University of Mexico (UNAM), Project code PAPIIT-DGAPA-IT101320: “Biorefinery for the production of volatile fatty acids, biogas and digestate from solid urban organic waste.”
Author information
Authors and Affiliations
Contributions
H.M.T. and O.G.B. conceived and designed the experiments; H.M.T. performed the experiments; H.M.T., O.G.B., and I.V.V. analyzed the data; H.M.T. and I.V.V. wrote and revised the manuscript; O.G.B., I.V.V., and A.D.M. supervised the work. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mosquera-Toscano, H.G., González-Barceló, O., Valdez-Vazquez, I. et al. Ethanol and Methane Production from the Organic Fraction of Municipal Solid Waste in a Two-Stage Process. Bioenerg. Res. 17, 634–645 (2024). https://doi.org/10.1007/s12155-023-10610-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-023-10610-w