Abstract
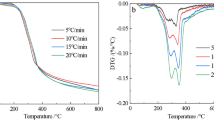
This research paper is about the kinetics of drying and pyrolysis processes of lulo (Solanum quitoense Lam.) peel powder, which was studied using thermogravimetric analysis (TG), differential scanning calorimetry (DSC), and mass spectrometry (MS). TG data was fitted using theoretical approximation according to the Newton model to obtain the kinetic parameters of drying, and the isoconversional methodology using Friedman’s method for the pyrolysis process. The results of each thermogram showed a relation between each other. In all of them, three characteristic stages were identified related to drying, pyrolysis, and carbonaceous matter. At the same time, there was a decomposition of the lignocellulosic biomass and light volatiles in the pyrolysis process. In the thermograms, three characteristic stages were identified: the first stage is the dehydration which ended at 120 °C, the second is the pyrolysis which is between 120 and 450 °C, and from this temperature, the third stage, carbonization, begins. In the pyrolysis stage, five peaks corresponding to independent reactions were identified; activation energy (Ea) and the reaction mechanism (f(α)) of each peak were calculated by means of master curves. After comparing the theoretical and experimental master plots, it was observed that the reaction mechanism corresponds to the Avrami-Erofeev model. Thermal analyses indicate that lulo peel is a potential waste for the production of coal for power purposes. It could be contributing to improve the management of waste and at the same time it could be used as a power supply or for water treatments such as activated carbon.






Similar content being viewed by others
References
Lo SL, Huang YF, Te Chiueh P, Kuan WH (2017) Microwave pyrolysis of lignocellulosic biomass. Energy Procedia 105:41–46
Bridgwater AV, Peacocke GVC (2000) Fast pyrolysis processes for biomass. Renew Sust Energ Rev 4:1–73
Wang S, Dai G, Yang H, Luo Z (2017) Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review. Prog. Energy Combust Sci 62:33–86
Forero DP, Orrego CE, Peterson DG, Osorio C (2015) Chemical and sensory comparison of fresh and dried lulo (Solanum quitoense Lam.) fruit aroma. Food Chem 169:85–91
Loaiza DIG, Santos LFO, Mahecha PV, Amariles HDV (2014) Cambios en las propiedades fisicoquímicas de frutos de lulo (So-lanum quitoense Lam.) cosechados en tres grados de madurez. Acta Agron 63:11–17
Laboratories PN, Beckman D (1991) Reviews developments in direct thermochemical liquefaction of. Energy Fuel 5:399–410
Zhao H, Li H, Song Q, Liu S, Yan J, Wang X (2019) Investigation on the physicochemical structure and gasification reactivity of nascent pyrolysis and gasification char prepared in the entrained flow reactor. Fuel 240:126–137
Zhao H, Li Y, Song Q, Liu S (2019) Investigation on the thermal behavior characteristics and products composition of four pulverized coals: its potential applications in coal cleaning. Int J Hydrogen Energ 44:23620–23638
Zhao H, Song Q, Liu S, Li Y, Wang X, Shu X (2018) Study on catalytic co-pyrolysis of physical mixture/staged pyrolysis characteristics of lignite and straw over an catalytic beds of char and its mechanism. Energy Convers Manag 161:13–26
Morais LC, Maia AAD, Guandique MEG, Rosa AH (2017) Pyrolysis and combustion of sugarcane bagasse. J Therm Anal Calorim 129:1813–1822
Lédé J (2012) Cellulose pyrolysis kinetics: an historical review on the existence and role of intermediate active cellulose. J Anal Appl Pyrolysis 94:17–32
Salcedo MJG, Contreras LK, García LA, Fernandez QA (2016) Modelado de la cinética de secado del afrecho de yuca (Manihot esculenta crantz). Rev Mex Ing Quim 15:883–891
Chen D, Zhang Y, Zhu X (2012) Drying kinetics of rice straw under isothermal and nonisothermal conditions: a comparative study by thermogravimetric analysis. Energy and Fuels 26:4189–4194
Vyazovkin S, Burnham AK, Criado JM, Pérez MLA, Popescu C, Sbirrazzuoli N (2011) ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19
Alves JLF, Silva JCG, Filho VFS, Alves RF, Galdino WVA, Andersen SLF, Sena RFS (2019) Determination of the bioenergy potential of Brazilian pine-fruit shell via pyrolysis kinetics, thermodynamic study, and evolved gas analysis. BioEnergy Res 12:168–183
Sánchez-Jiménez PE, Pérez-Maqueda LA, Perejón A, Criado JM (2010) Generalized kinetic master plots for the thermal degradation of polymers following a random scission mechanism. J Phys Chem A 114:7868–7876
Romero Millán LM, Sierra Vargas FE, Nzihou A (2017) Kinetic analysis of tropical lignocellulosic agrowaste pyrolysis. Bioenergy Res 10:832–845
Kar Y (2018) Environmental Effects Pyrolysis of waste pomegranate peels for bio-oil production. Energy Sources, Part A Recover. Util Environ Eff 00:1–10
AOAC, Official methods of analysis (1995) Assoc Anal Communities 1:141–144
Criado JM, Sánchez JPE, Pérez MLA (2008) Critical study of the isoconversional methods of kinetic analysis. J Therm Anal Calorim 92:199–203
Omrani A, Rostami AA, Ravari F (2013) Advanced isoconversional and master plot analyses on solid-state degradation kinetics of a novel nanocomposite. J Therm Anal Calorim 111:677–683
Sanchez SL, López GD, Villaseñor J, Sánchez P, Valverde JL (2012) Thermogravimetric-mass spectrometric analysis of lignocellulosic and marine biomass pyrolysis. Bioresour Technol 109:163–172
Greenhalf CE, Nowakowski DJ, Bridgwater AV, Titiloye J, Yates N, Riche A, Shield I (2012) Thermochemical characterization of straws and high yielding perennial grasses. Ind Crop Prod 36:449–459
Omar R, Idris A, Yunus R, Khalid K, Isma MIA (2011) Characterization of empty fruit bunch for microwave-assisted pyrolysis. Fuel 90:1536–1544
Li D, Chen L, Yi X, Zhang X, Ye N (2010) Pyrolytic characteristics and kinetics of two brown algae and sodium alginate. Bioresour Technol 101:7131–7136
Mishra RK, Mohanty K (2018) Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour Technol 251:63–74
Sait HH, Hussain A, Salema AA, Ani FN (2012) Pyrolysis and combustion kinetics of date palm biomass using thermogravimetric analysis. Bioresour Technol 118:382–389
Cai J, Xu D, Dong Z, Yu X, Yang Y, Banks SW, Bridgwater AV (2018) Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: case study of corn stalk. Renew Sust Energ Rev 82:2705–2715
Yang X, Zhao Y, Li R, Wu Y, Yang M (2018) Thermochimica Acta A modified kinetic analysis method of cellulose pyrolysis based on TG–FTIR technique. Thermochim Acta 665:20–27
Mattos B, Lazzarotto M, Magalhães WLE, Gatto DA (2015) Thermal tools to evaluation of decayed and weathered wood polymer composites prepared by in situ polymerization. J Therm Anal Calorim 121:1263–1271
Yang H, Yan R, Chen H, Lee DH, Zheng C (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788
Comesaña JA, Nieströj M, Granada E, Szlek A (2013) TG-DSC analysis of biomass heat capacity during pyrolysis process. J Energy Inst 86:153–159
Cai J, Chen S (2008) Determination of drying kinetics for biomass by thermogravimetric analysis under nonisothermal condition. Dry Technol 26:1464–1468
Bruijn TJW, Jong WA, Berg WJ (1981) Kinetic parameters in Avrami-Erofeev type reactions from isothermal and non-isothermal experiments. Thermochim Acta 45:315–325
Zlatanović S, Ostojić S, Micić D, Rankov S, Dodevska M, Vukosavljević P, Gorjanović S (2019) Thermal behaviour and degradation kinetics of apple pomace flours. Thermochim Acta 673:17–25
Saavedra LMZ, Alvarez SC, Esneider AMA, Toxqui TA, Pérez GSA, Ruiz CMA (2012) Towards an improved calorimetric methodology for glass transition temperature determination in amorphous sugars. J Food 10:258–267
Hurtta M, Pitkänen I, Knuutinen J (2004) Melting behaviour of D-sucrose, D-glucose and D-fructose. Carbohydr Res 339:2267–2273
Chen T, Wu J, Zhang J, Wu J, Sun L (2014) Gasification kinetic analysis of the three pseudocomponents of biomass-cellulose, semicellulose and lignin. Bioresour Technol 153:223–229
Wongsiriamnuay T, Tippayawong N (2010) Non-isothermal pyrolysis characteristics of giant sensitive plants using thermogravimetric analysis. Bioresour Technol 101:5638–5644
Jia C, Chen J, Bai J, Yang X, Song S, Wang Q (2018) Kinetics of the pyrolysis of oil sands based upon thermogravimetric analysis. Thermochim Acta 666:66–74
Collard FX, Blin J (2014) A review on pyrolysis of biomass constituents: mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev 38:594–608
Stefanidis SD, Kalogiannis KG, Iliopoulou EF, Michailof CM, Pilavachi PA, Lappas AA (2014) A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J Anal Appl Pyrolysis 105:143–150
Jiang G, Nowakowski DJ, Bridgwater AV (2010) A systematic study of the kinetics of lignin pyrolysis. Thermochim Acta 498:61–66
Wang X, Hu M, Hu W, Chen Z, Liu S, Hu Z, Xiao B (2016) Thermogravimetric kinetic study of agricultural residue biomass pyrolysis based on combined kinetics. Bioresour Technol 219:510–520
Burnham AK, Zhou X, Broadbelt LJ (2015) Critical review of the global chemical kinetics of cellulose thermal decomposition. Energy and Fuels 29:2906–2918
Chen Z, Hu M, Zhu X, Guo D, Liu S, Hu Z, Xiao B, Wang J, Laghari M (2015) Characteristics and kinetic study on pyrolysis of five lignocellulosic biomass via thermogravimetric analysis. Bioresour Technol 192:441–450
White JE, Catallo WJ, Legendre BL (2011) Biomass pyrolysis kinetics: a comparative critical review with relevant agricultural residue case studies. J Anal Appl Pyrolysis 91:1–33
Acknowledgments
The authors gratefully acknowledge the use of laboratory infrastructure from the Universidad Nacional de Colombia, Sede Palmira, and the thermal analysis research laboratory of Autónoma de Occidente University, Cali-Colombia.
Funding
This work received financial support from the Universidad Nacional de Colombia with code QUIPU 202010013208.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 270 kb)
Rights and permissions
About this article
Cite this article
Chacon, W.D.C., Valencia, G.A., Rojas, G.M.A. et al. Drying and Pyrolysis of Lulo Peel: Non-Isothermal Analysis of Physicochemical, Kinetics, and Master Plots. Bioenerg. Res. 13, 927–938 (2020). https://doi.org/10.1007/s12155-020-10127-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10127-6