Abstract
Objective
Sialadenitis and salivary gland disorders are complications of radioactive iodine therapy (RAIT) that affect the quality of life of patients with differentiated thyroid cancer (DTC). The current study aimed to provide evidence on the protective effect of apitherapy on salivary gland function during RAIT in patients with DTC.
Methods
In total, 120 patients with DTC who underwent total thyroidectomy were divided into the apitherapy group (group A, n = 60) and the control group (group B, n = 60). Group A received 2.5 g of acacia honey three times daily after each meal during admission for RAIT. Statistical analyses were performed using the Saxon test (which is used to evaluate saliva volume) and salivary gland scintigraphy (which is applied to assess maximum uptake ratio and washout ratio).
Results
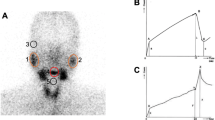
Compared with group B, group A presented with a more significantly positive change in the rate of amount of saliva before and after treatment (P < 0.01). Group B presented a significant decrease in the maximum uptake ratio of the bilateral parotid and submandibular glands on salivary gland scintigraphy (P < 0.05) and washout ratio of all salivary glands (P < 0.05). Group A did not present significant differences in the maximum uptake ratio and washout ratio.
Conclusions
Apitherapy can have protective effects against salivary gland disorder associated with RAIT in patients with DTC.







Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
References
Pacini F, Fuhrer D, Elisei R, et al. 2022 ETA Consensus Statement: What are the indications for post-surgical radioiodine therapy in differentiated thyroid cancer? Eur Thyroid J. 2022;11(1): e210046.
Sparano C, Moog S, Hadoux J, et al. Strategies for radioiodine treatment: what’s New. Cancers (Basel). 2022;14(15):3800.
Ciarallo A, Rivera J. Radioactive iodine therapy in differentiated thyroid cancer: 2020 update. AJR Am J Roentgenol. 2020;215(2):285–91.
Lee SW. SPECT/CT in the treatment of differentiated thyroid cancer. Nucl Med Mol Imaging. 2017;51(4):297–303.
Liu J, Liu Y, Lin Y, Liang J. Radioactive iodine-refractory differentiated thyroid cancer and redifferentiation therapy. Endocrinol Metab (Seoul). 2019;34(3):215–25.
Adramerinas M, Andreadis D, Vahtsevanos K, Poulopoulos A, Pazaitou-Panayiotou K. Sialadenitis as a complication of radioiodine therapy in patients with thyroid cancer: where do we stand? Hormones (Athens). 2021;20(4):669–78.
Auttara-Atthakorn A, Sungmala J, Anothaisintawee T, Reutrakul S, Sriphrapradang C. Prevention of salivary gland dysfunction in patients treated with radioiodine for differentiated thyroid cancer: a systematic review of randomized controlled trials. Front Endocrinol (Lausanne). 2022;13: 960265.
Noaparast Z, Hosseinimehr SJ. Radioprotective agents for the prevention of side effects induced by radioiodine-131 therapy. Future Oncol. 2013;9(8):1145–59.
Christou A, Papastavrou E, Merkouris A, Frangos S, Tamana P, Charalambous A. Clinical studies of nonpharmacological methods to minimize salivary gland damage after radioiodine therapy of differentiated thyroid carcinoma: systematic review. Evid Based Complement Alternat Med. 2016;2016:6795076.
Nakada K, Ishibashi T, Takei T, et al. Does lemon candy decrease salivary gland damage after radioiodine therapy for thyroid cancer? J Nucl Med. 2005;46(2):261–6.
Münstedt K, Momm F, Hübner J. Honey in the management of side effects of radiotherapy or radio/chemotherapy-induced oral mucositis. A systematic review. Complement Ther Clin Pract. 2019;34:145–52.
Xu JL, Xia R, Sun ZH, et al. Effects of honey use on the management of radio/chemotherapy-induced mucositis: a meta-analysis of randomized controlled trials. Int J Oral Maxillofac Surg. 2016;45(12):1618–25.
Yang C, Gong G, Jin E, et al. Topical application of honey in the management of chemo/radiotherapy-induced oral mucositis: a systematic review and network meta-analysis. Int J Nurs Stud. 2019;89:80–7.
Thomsen M, Vitetta L. Adjunctive treatments for the prevention of chemotherapy- and radiotherapy-induced mucositis. Integr Cancer Ther. 2018;17(4):1027–47.
Khanjani Pour-Fard-Pachekenari A, Rahmani A, Ghahramanian A, Asghari Jafarabadi M, Onyeka TC, Davoodi A. The effect of an oral care protocol and honey mouthwash on mucositis in acute myeloid leukemia patients undergoing chemotherapy: a single-blind clinical trial. Clin Oral Investig. 2019;23(4):1811–21.
Amanat A, Ahmed A, Kazmi A, Aziz B. The effect of honey on radiation-induced oral mucositis in head and neck cancer patients. Indian J Palliat Care. 2017;23(3):317–20.
Mandal MD, Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed. 2011;1(2):154–60.
Molan P, Rhodes T. Honey: a biologic wound dressing. Wounds. 2015;27(6):141–51.
Burlando B, Cornara L. Honey in dermatology and skin care: a review. J Cosmet Dermatol. 2013;12(4):306–13.
da Silva PM, Gauche C, Gonzaga LV, Costa AC, Fett R. Honey: chemical composition, stability and authenticity. Food Chem. 2016;196:309–23.
Bardy J, Molassiotis A, Ryder WD, et al. A double-blind, placebo-controlled, randomised trial of active Manuka honey and standard oral care for radiation-induced oral mucositis. Br J Oral Maxillofac Surg. 2012;50(3):221–6.
Ono Minagi H, Yamanaka Y, Sakai T. Evaluation of the Saxon test for patients with hyposalivation without Sjögren’s syndrome. J Oral Rehabil. 2020;47(12):1550–6.
Nakayama M, Okizaki A, Takahashi K. A randomized controlled trial for the effectiveness of aromatherapy in decreasing salivary gland damage following radioactive iodine therapy for differentiated thyroid cancer. Biomed Res Int. 2016;2016:9509810.
Mărgăoan R, Topal E, Balkanska R, et al. Monofloral honeys as a potential source of natural antioxidants, minerals and medicine. Antioxidants (Basel). 2021;10(7):1023.
Gül A, Pehlivan T. Antioxidant activities of some monofloral honey types produced across Turkey. Saudi J Biol Sci. 2018;25(6):1056–65.
Crăciun ME, Pârvulescu OC, Donise AC, Dobre T, Stanciu DR. Characterization and classification of Romanian acacia honey based on its physicochemical parameters and chemometrics. Sci Rep. 2020;10(1):20690.
Chua LS, Rahaman NL, Adnan NA, Eddie Tan TT. Antioxidant activity of three honey samples in relation with their biochemical components. J Anal Methods Chem. 2013;2013: 313798.
Mukai K, Koike M, Nakamura S, et al. Evaluation of the effects of a combination of Japanese honey and hydrocolloid dressing on cutaneous wound healing in male mice. Evid Based Complement Alternat Med. 2015;2015: 910605.
Nakajima Y, Nakano Y, Fuwano S, et al. Effects of three types of Japanese honey on full-thickness wound in mice. Evid Based Complement Alternat Med. 2013;2013: 504537.
Sawazaki T, Nakajima Y, Urai T, et al. Efficacy of honeydew honey and blossom honey on full-thickness wound healing in mice. Wounds. 2018;30(7):197–204.
Wilson KF, Meier JD, Ward PD. Salivary gland disorders. Am Fam Physician. 2014;89(11):882–8.
Bobiş O, Dezmirean DS, Moise AR. Honey and diabetes: the importance of natural simple sugars in diet for preventing and treating different type of diabetes. Oxid Med Cell Longev. 2018;2018:4757893.
Samarghandian S, Farkhondeh T, Samini F. Honey and health: a review of recent clinical research. Pharmacogn Res. 2017;9(2):121–7.
Funding
We have received no funding or other financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nomura, K., Nakayama, M. & Okizaki, A. Effects of apitherapy against salivary gland disorder after radioactive iodine therapy for differentiated thyroid cancer. Ann Nucl Med 37, 462–469 (2023). https://doi.org/10.1007/s12149-023-01845-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-023-01845-w