Abstract
Objective
The γ-Ray Evaluation with iodoamphetamine for Cerebral Blood Flow Assessment (REICA) is a new method for quantifying cerebral blood flow (CBF) using single-photon emission computed tomography (SPECT) and [123I]N-isopropyl-p-iodoamphetamine (123I-IMP). The present study aimed to validate the REICA method using data including acetazolamide challenge test.
Methods
The REICA and Graph-Plot (GP) methods were used to calculate mean CBF (mCBF) for 92 acquisitions (rest: 57, stress: 35) and cerebrovascular reactivity (CVR) in 33 patients. To obtain stress data, 15 mg/kg of acetazolamide was injected intravenously 10 min before the administration of 123I-IMP, and blood samples were collected under the same conditions as rest data. The reference standard was the Autoradiograph (ARG) method using arterial blood sampling, and the accuracy of the REICA method was analyzed by comparing it with each method.
Results
For mCBF, the correlation coefficients (r) were 0.792 for the REICA method and 0.636 for the GP method. For CVR, r values were 0.660 for the REICA method and 0.578 for the GP method. In both acquisitions, the REICA method had a stronger correlation with the ARG method than the GP method. For mCBF, there was a significant difference in the correlation coefficient between the two correlation coefficients (p < 0.01).
Conclusions
The REICA method was more accurate than the GP method in quantifying CBF and closer to the ARG method. The REICA method, which is a noninvasive method of cerebral blood flow quantification using 123I-IMP, has great medical usefulness.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Single-photon emission computed tomography (SPECT), which evaluates ischemic cerebrovascular disease [1] and diagnoses dementia [2], can aid in determining treatment strategies as well as therapeutic and surgical effects through analyses of cerebral blood flow (CBF) [3]. Furthermore, the combination of “stress” data obtained during CBF loading with acetazolamide (ACZ) and “rest” data during resting CBF can be used to calculate cerebrovascular reactivity (CVR), which can in turn be used to predict the patients at high risk for perioperative hyperperfusion [4,5,6,7,8,9].
There are different types of cerebral blood flow SPECT tracers, such as 99mTc-hexamethylpropyleneamine oxime (99mTc-HMPAO) and 99mTc-ethyl cysteinate dimer (99mTc-ECD). [123I]N-isopropyl-p-iodoamphetamine (123I-IMP) is an ideal tracer in that it does not underestimate regional cerebral blood flow (rCBF) at high blood flow values [10].
In the quantification of cerebral blood flow by SPECT, the Autoradiograph (ARG) method [11] is a simplified version of the Table Look Up method [12] based on a one-tissue, two-compartment model. Currently, the ARG method, which requires arterial blood sampling, is the standard method for CBF quantification using 123I-IMP.
Graph-Plot (GP) method [13], another method of CBF quantification that does not use arterial blood sampling has some serious methodological flaws. It is not appropriate to use pulmonary artery measurements as the input function [14].
Recently, a new CBF quantification method using SPECT and 123I-IMP has been proposed [14]. The γ-Ray Evaluation with iodoamphetamine for Cerebral blood flow Assessment (REICA) method does not require invasive arterial blood sampling, nor does it require the use of a regression formula. Moreover, the method removes bias due to the variable pulmonary tracer retention by accounting for it within the equation. However, there are only two reports on the REICA method [14, 15], and they only validated the REICA method on rest data using a single γ-camera, and no reports of stress data obtained under ACZ loading have been published yet.
Therefore, in the present study, we aimed to validate the REICA method.
Materials and methods
Participants
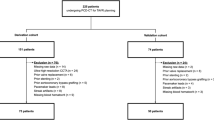
A total of 59 patients (22 males and 37 females; mean age 59.7 ± 17.5) who underwent simultaneous GP and ARG procedures between August 2018 and November 2019 were included in the study (Table 1). Rest/stress examinations of the same patient were performed at intervals of 7 days or less. Diseases among the included participants were Moyamoya disease (18 patients), middle cerebral artery stenosis or occlusion (20 patients), carotid artery stenosis or occlusion (16 patients), and other vascular disorders (5 patients).
For the clinical images in this study, care was taken to protect personal information to prevent the identification of participants. This study was conducted in accordance with the Declaration of Helsinki 1975, as revised in 2000. The Ethics Review Committee of National Hospital Organization Kyushu Medical Center approved the utilization of data (Approval no. 19C203) and waived the need for patient consent for the utilization of existing data (the retrospective study).
Data acquisition
The dynamic planar protocol (Fig. 1) was used as previously reported [14]. Dynamic frontal planar scans (2 s/frame, 60 frames, 3.9 mm/pixel) were acquired using a γ-camera (Siemens E.CAM, Siemens Medical Solutions, Erlangen, Germany) equipped with a low-energy high-resolution collimator from intravenous administration of a 167 MBq 123I-IMP. The rest and stress data were acquired using the same γ-camera. Arterial blood samples were measured for γ-ray radiation counts using a well-type scintillation measurement device (NDW-351F; Hitachi Aloka Medical, Ltd.). The arterial blood samples were taken 10 min after the 123I-IMP administration, and the input function values were obtained from the average of four measurements of radioactivity concentration per 1.0 g. To obtain stress data, 15 mg/kg of ACZ was injected intravenously 10 min before the administration of 123I-IMP, and blood samples were collected under the same conditions as rest data. The image reconstruction method is filtered back projection, the reconstruction filter is ramp, and the preprocessing filter is Butterworth (cutoff 0.35 cycle/cm; order 8). Chang’s method was adopted for attenuation correction, and the attenuation coefficient was set to 0.07/cm. Scattering correction was not performed.
Data analysis
The REICA method was used for data analysis in this study. Regions of interest (ROIs) were selected for the pulmonary artery trunk, brain, and lungs. The time-activity curves (TACs) calculated by the ROI was smoothed with a low-pass filter to reduce statistical noise. After that, a time zero adjustment was performed when sharp increases in activity were observed [13, 16]. In this study, \(\left( {\frac{{\mathop \smallint \nolimits_{0}^{t} C_{{\text{r}}} \left( \tau \right){\text{d}}\tau }}{l\left( t \right)},\;\frac{{C_{{\text{b}}} \left( t \right)}}{\lambda l\left( t \right)}} \right)\) was plotted, and the slope of the linear section was determined. Cr(t) denotes tracer activity in the pulmonary artery, l(t) denotes activity in the lungs, Cb(t) denotes activity in the brain, and λ denotes lipophilic fraction of 123I-IMP [14, 15].
The ARG method was performed using the built-in software (Syngo MI Applications®, Siemens Medical Solutions, Erlangen, Germany). The volume of distribution (Vd) value was set to 40. For the quantitative SPECT images obtained by the ARG method, ROIs were set on the normal side of the brain parenchyma nuclei in the basal ganglia slice, and mean CBF (mCBF) by the ARG method was obtained.
The GP method was analyzed using dedicated software Hayabusa (AZE VirtualPlace Hayabusa®, AZE, Tokyo, Japan). For the analysis, ROIs were set in the pulmonary artery and basal ganglia, respectively. For each ROI, the standardized F (SFR, F = Cerebral blood flow index) was obtained using the TACs of the pulmonary artery as the input function and the TACs of the brain as the output function. The mCBF was calculated from SFR using a conversion formula. The conversion equation for the GP method is as follows [17]:
The CVR for stress data obtained after injection of ACZ is calculated using the following equation [6]:
The mCBF value acquired using the ARG method was used as the reference standard.
Statistical analysis
The data, including significance levels, were analyzed using Excel® 2016 (Microsoft Corporation, Redmond, WA, USA).
Results
mCBF
Figure 2 shows the relationship between mCBF measured using the REICA and ARG method and mCBF measured using the GP and ARG method for 92 acquisition (rest: 57, stress: 35). The linear regression and correlation coefficients (r) were as follows: REICA and ARG method, y = 1.17 x−1.59, r = 0.792, p = 5.96 × 10–21; GP and ARG method, y = 0.634 x + 25.6, r = 0.636, p = 1.01 × 10–11. Furthermore, the difference between the two correlation coefficients was significant at p = 7.30 × 10–3, indicating that the REICA method had a stronger correlation with the ARG method than the GP method. The slope of the linear regression for the REICA method was closer to 1.0 and the intercept to 0.0 than those of the linear regression for the GP method.
CVR
Figure 3 shows the results of the CVRs of REICA and ARG, of GP and ARG for the data of 33 patients. The respective linear regression and correlation coefficients were as follows: REICA and ARG method, y = 1.11 x + 6.93, r = 0.660, p = 2.96 × 10–5; GP and ARG method, y = 0.642 x + 4.25, r = 0.578, p = 4.28 × 10–4. Compared with the GP method, the REICA method yielded positive CVR values, and the slope of the linear regression equation was close to 1.0.
Discussion
The present study is the first to verify the REICA method for calculating CBF and CVR using data including acetazolamide challenge test. Our findings indicated that, especially for mCBF, there was a significant difference between the two correlation coefficients (p < 0.01), proving the high accuracy of the REICA method. Adding this study using Siemens SPECT machine to the previous studies using GE machine, we hope that REICA method can be generalized. In addition, during acetazolamide administration, the blood flow tracer is rapidly transferred to the brain. The CBV is about 3–5% [18], and the effect of the change in CBV on the mCBF measurement measured by the REICA and GP methods would be minimal.
The linear regression equation for mCBF determined using the GP method exhibited a low slope (0.634, REICA: 1.17) and high intercept (25.6, REICA: − 1.59), suggesting that mCBF was overestimated when low. The conversion formula used in the GP method when calculating mCBF was mainly constructed from the rest dataset [13], which may have caused a discrepancy in the quantitative values in high blood flow, and the mCBF values in the stress data are likely to be underestimated. In other words, the use of a conversion formula that is not appropriate for each condition increases the possibility of a mCBF error.
Furthermore, in the calculation of CVR using Eq. (1), overestimation of mCBFrest and underestimation of mCBFstress can result in further underestimation of CVR, which can lead to the incorrect judgment that there is a risk of overperfusion. Previous studies have reported that CVR obtained based on SPECT analysis of CBF using ACZ loading can help to identify patients at high risk of recurrent stroke [5, 6], and to predict hyperperfusion in patients undergoing carotid endarterectomy [7, 8] and carotid artery stenting [9, 10]. These studies highlight the importance of this index in the perioperative complication cycle.
REICA method may be susceptible to body weight and the distance between the camera and body. The input function of a heavier person may be underestimated due to the attenuation of the γ-Ray, which would lead to the overestimation of CBF. A parallel hole collimator would not affect the counts, however, the longer distance between the camera and the body would make the image blur, which may lead to underestimation of the counts. The same phenomenon would be considered to be occurred in the GP method and the Gjedde–Patlak–Matsuda method. Further studies would be needed to confirm this speculation.
The present study has a limitation. The number of patients, especially who underwent both rest and stress tests were not large, because the participants were recruited from a single hospital.
Conclusion
The present validation was performed by adding REST data to the stress data, which has not been reported so far in the REICA method. It was found that the quantification of CBF by the REICA method was more accurate than the GP method, which was closer to the ARG method. Thus, our findings highlight the medical potential of REICA method for noninvasive quantification of CBF using 123I-IMP.
References
Kim KM, Watabe H, Hayashi T, Hayashida K, Katafuchi T, Enomoto N, Ogura T, Shidahara M, Takikawa S, Eberl S, Nakazawa M, Iida H. Quantitative mapping of basal and vasareactive cerebral blood flow using split-dose 123I-iodoamphetamine and single photon emission computed tomography. Neuroimage. 2006;33(4):1126–35. https://doi.org/10.1016/j.neuroimage.2006.06.064.
Ferrando R, Damian A. Brain SPECT as a biomarker of neurodegeneration in dementia in the era of molecular imaging: still a valid option? Front Neurol. 2021;10(12): 629442. https://doi.org/10.3389/fneur.2021.629442.
Mizumura S, Nakagawara J, Takahashi M, Kumita S, Cho K, Nakajo H, Toba M, Kumazaki T. Three-dimensional display in staging hemodynamic brain ischemia for JET study: objective evaluation using SEE analysis and 3D-SSP display. Ann Nucl Med. 2004;18(1):13–21. https://doi.org/10.1007/BF02985609.
Ogasawara K, Ogawa A, Yoshimoto T. Cerebrovascular reactivity to acetazolamide and outcome in patients with symptomatic internal carotid or middle cerebral artery occlusion: a xenon-133 single-photon emission computed tomography study. Stroke. 2002;33(7):1857–62. https://doi.org/10.1161/01.str.0000019511.81583.a8.
Kuroda S, Houkin K, Kamiyama H, Mitsumori K, Iwasaki Y, Abe H. Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: can acetazolamide test predict it? Stroke. 2001;32(9):2110–6. https://doi.org/10.1161/hs0901.095692.
Oshida S, Ogasawara K, Saura H, Yoshida K, Fujiwara S, Kojima D, Kobayashi M, Yoshida K, Kubo Y, Ogawa A. Does preoperative measurement of cerebral blood flow with acetazolamide challenge in addition to preoperative measurement of cerebral blood flow at the resting state increase the predictive accuracy of development of cerebral hyperperfusion after carotid endarterectomy? Results from 500 cases with brain perfusion single-photon emission computed tomography study. Neurol Med Chir (Tokyo). 2015;55(2):141–8. https://doi.org/10.2176/nmc.oa.2014-0269.
Hosoda K, Kawaguchi T, Shibata Y, Kamei M, Kidoguchi K, Koyama J, Fujita S, Tamaki N. Cerebral vasoreactivity and internal carotid artery flow help to identify patients at risk for hyperperfusion after carotid endarterectomy. Stroke. 2001;32(7):1567–73. https://doi.org/10.1161/01.str.32.7.1567.
Kaku Y, Yoshimura S, Kokuzawa J. Factors predictive of cerebral hyperperfusion after carotid angioplasty and stent placement. AJNR Am J Neuroradiol. 2004;25(8):1403–8.
Ogasawara K, Yukawa H, Kobayashi M, Mikami C, Konno H, Terasaki K, Inoue T, Ogawa A. Prediction and monitoring of cerebral hyperperfusion after carotid endarterectomy by using single-photon emission computerized tomography scanning. J Neurosurg. 2003;99(3):504–10. https://doi.org/10.3171/jns.2003.99.3.0504.
Kameyama M. Lassen’s equation is a good approximation of permeability-surface model: new α values for 99mTc-HMPAO and 99mTc-ECD. J Cereb Blood Flow Metab. 2014;34(7):1157–61. https://doi.org/10.1038/jcbfm.2014.64.
Iida H, Itoh H, Nakazawa M, Hatazawa J, Nishimura H, Onishi Y, Uemura K. Quantitative mapping of regional cerebral blood flow using iodine-123-IMP and SPECT. J Nucl Med. 1994;35(12):2019–30.
Iida H, Itoh H, Bloomfield PM, Munaka M, Higano S, Murakami M, Inugami A, Eberl S, Aizawa Y, Kanno I, et al. A method to quantitate cerebral blood flow using a rotating gamma camera and iodine-123 iodoamphetamine with one blood sampling. Eur J Nucl Med. 1994;21(10):1072–84. https://doi.org/10.1007/BF00181062.
Okamoto K, Ushijima Y, Okuyama C, Nakamura T, Nishimura T. Measurement of cerebral blood flow using graph plot analysis and I-123 iodoamphetamine. Clin Nucl Med. 2002;27(3):191–6. https://doi.org/10.1097/00003072-200203000-00009.
Kameyama M, Watanabe K. A new non-invasive graphical method for quantification of cerebral blood flow with [123I] IMP. Ann Nucl Med. 2018;32(9):620–6. https://doi.org/10.1007/s12149-018-1282-8.
Maruko M, Kameyama M, Sakai J, Shirasaki S, Fujiwara H. The REICA method for quantification of cerebral blood flow is less affected by lung washout of [123I] IMP than the graph-plot method. Ann Nucl Med. 2020;34(10):757–61. https://doi.org/10.1007/s12149-020-01499-y.
Matsuda H, Tsuji S, Shuke N, Sumiya H, Tonami N, Hisada K. A quantitative approach to technetium-99m hexamethylpropylene amine oxime. Eur J Nucl Med. 1992;19(3):195–200. https://doi.org/10.1007/BF00173281.
Ishii K, Uemura T, Miyamoto N, Yoshikawa T, Yamaguchi T, Ashihara T, Ohtani Y. Regional cerebral blood flow in healthy volunteers measured by the graph plot method with iodoamphetamine SPECT. Ann Nucl Med. 2011;25(4):255–60. https://doi.org/10.1007/s12149-010-0451-1.
Okazawa H, Yamauchi H, Sugimoto K, Toyoda H, Kishibe Y, Takahashi M. Effects of acetazolamide on cerebral blood flow, blood volume, and oxygen metabolism: a positron emission tomography study with healthy volunteers. J Cereb Blood Flow Metab. 2001;21(12):1472–9. https://doi.org/10.1097/00004647-200112000-00012.
Acknowledgements
The authors would like to thank Mr. Kazuki Shimomuta (present affiliation: Itoshima Medical Association Hospital) and Mr. Suguru Kawamuro (present affiliation: Fukuoka Kieikai Hospital), Radiological technologists of the National Hospital Organization Kyushu Medical Center for collecting data, Mr. Tomohiko Aso, a radiological technologist of the National Cancer Center for supporting this study, and Editage (www.editage.com) for English language editing of this manuscript.
Funding
YM and NT received research funding from Nihon Medi-Physics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MK is listed as an inventor on a patent application (2019–074369, 2017–199618) related to this work submitted by Nihon Medi-Physics to the Japan Patent Office. MK received research funding from Nihon Medi-Physics and Fujifilm Toyama Chemistry, which supply [123I] IMP in Japan.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miyazaki, Y., Kameyama, M., Nakamizo, A. et al. Validity of the γ-Ray Evaluation with iodoamphetamine for Cerebral Blood Flow Assessment (REICA) method for quantification of cerebral blood flow including acetazolamide challenge test. Ann Nucl Med 36, 279–284 (2022). https://doi.org/10.1007/s12149-021-01700-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-021-01700-w