Abstract
Objective
The diagnostic performance of 18F-FDG PET for detecting regional lymph node metastasis in resectable lung cancer is variable, and its sensitivity for adenocarcinoma is even lower. We aimed to evaluate the value of 18F-FDG PET-derived features in predicting pathological lymph node metastasis in patients with lung adenocarcinoma.
Methods
We retrospectively analyzed pretreatment 18F-FDG PET-derived features of 126 lung adenocarcinoma patients who underwent curative surgery. A logistic regression model was used to analyze the association between study variables and pathological regional lymph node status obtained from the curative surgery. Furthermore, Cox regression analysis was used to test the effect of the study variables on survival outcomes, including disease-free survival (DFS) and overall survival (OS).
Results
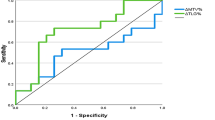
The primary tumor entropy (OR = 1.7, p = 0.014) and visual interpretation of regional nodes via 18F-FDG PET (OR = 2.5, p = 0.026) independently predicted pathological regional lymph node metastasis. The areas under the receiver-operating-characteristic curves were 0.631, 0.671, and 0.711 for visual interpretation, primary tumor entropy, and their combination, respectively. Based on visual interpretation, a primary tumor entropy ≥ 3.0 improved the positive predictive value of positive visual interpretation from 51.2% to 63.0%, whereas an entropy < 3.0 improved the negative predictive value of negative visual interpretation from 75.3% to 82.6%. In cases with positive visual interpretation and low entropy, or negative visual interpretation and high entropy, the nodal metastasis rates were approximately 30%. In the survival analyses, the primary tumor entropy was also independently associated with DFS (HR = 2.7, p = 0.001) and OS (HR = 4.8, p = 0.001).
Conclusions
Our preliminary results show that the primary tumor entropy may improve 18F-FDG PET visual interpretation in predicting pathological nodal metastasis in lung adenocarcinoma, and may also show a survival prognostic value. This versatile biomarker may facilitate tailored therapeutic strategies for patients with resectable lung adenocarcinoma.



Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health. 2019;85:8.
Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51.
Asamura H, Chansky K, Crowley J, Goldstraw P, Rusch VW, Vansteenkiste JF, et al. The international association for the study of lung cancer lung cancer staging project: proposals for the revision of the N descriptors in the forthcoming 8th edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10:1675–84.
Zhang Y, Hu X, Liu D, Wang R, Sun X, Peng Z, et al. Effectiveness of neoadjuvant chemotherapy on the survival outcomes of patients with resectable non-small-cell lung cancer: a meta-analysis of randomized controlled trials. Surg Oncol. 2021;38:101590.
Birim O, Kappetein AP, Stijnen T, Bogers AJ. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann Thorac Surg. 2005;79:375–82.
Gomez-Caro A, Boada M, Cabanas M, Sanchez M, Arguis P, Lomena F, et al. False-negative rate after positron emission tomography/computer tomography scan for mediastinal staging in cI stage non-small-cell lung cancer. Eur J Cardiothorac Surg. 2012;42:93–100 (discussion 100).
Bille A, Pelosi E, Skanjeti A, Arena V, Errico L, Borasio P, et al. Preoperative intrathoracic lymph node staging in patients with non-small-cell lung cancer: accuracy of integrated positron emission tomography and computed tomography. Eur J Cardiothorac Surg. 2009;36:440–5.
Bille A, Okiror L, Skanjeti A, Errico L, Arena V, Penna D, et al. Evaluation of integrated positron emission tomography and computed tomography accuracy in detecting lymph node metastasis in patients with adenocarcinoma vs squamous cell carcinoma. Eur J Cardiothorac Surg. 2013;43:574–9.
Schmidt-Hansen M, Baldwin DR, Hasler E, Zamora J, Abraira V, Roque IFM. PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer. Cochrane Database Syst Rev. 2014;2014:CD009519.
Serra Fortuny M, Gallego M, Berna L, Monton C, Vigil L, Masdeu MJ, et al. FDG-PET parameters predicting mediastinal malignancy in lung cancer. BMC Pulm Med. 2016;16:177.
Ouyang ML, Tang K, Xu MM, Lin J, Li TC, Zheng XW. Prediction of occult lymph node metastasis using tumor-to-blood standardized uptake ratio and metabolic parameters in clinical N0 lung adenocarcinoma. Clin Nucl Med. 2018;43:715–20.
Ouyang ML, Xia HW, Xu MM, Lin J, Wang LL, Zheng XW, et al. Prediction of occult lymph node metastasis using SUV, volumetric parameters and intratumoral heterogeneity of the primary tumor in T1–2N0M0 lung cancer patients staged by PET/CT. Ann Nucl Med. 2019;33:671–80.
Han S, Woo S, Suh CH, Kim YJ, Oh JS, Lee JJ. A systematic review of the prognostic value of texture analysis in 18F-FDG PET in lung cancer. Ann Nucl Med. 2018;32:602–10.
Park S, Ha S, Lee SH, Paeng JC, Keam B, Kim TM, et al. Intratumoral heterogeneity characterized by pretreatment PET in non-small cell lung cancer patients predicts progression-free survival on EGFR tyrosine kinase inhibitor. PLoS ONE. 2018;13:e0189766.
Chen YH, Wang TF, Chu SC, Lin CB, Wang LY, Lue KH, et al. Incorporating radiomic feature of pretreatment 18F-FDG PET improves survival stratification in patients with EGFR-mutated lung adenocarcinoma. PLoS ONE. 2020;15:e0244502.
Caswell DR, Swanton C. The role of tumour heterogeneity and clonal cooperativity in metastasis, immune evasion and clinical outcome. BMC Med. 2017;15:133.
Detterbeck FC, Chansky K, Groome P, Bolejack V, Crowley J, Shemanski L, et al. The IASLC lung cancer staging project: methodology and validation used in the development of proposals for revision of the stage classification of NSCLC in the forthcoming (eighth) edition of the TNM classification of lung cancer. J Thorac Oncol. 2016;11:1433–46.
Kim YK, Lee KS, Kim BT, Choi JY, Kim H, Kwon OJ, et al. Mediastinal nodal staging of nonsmall cell lung cancer using integrated 18F-FDG PET/CT in a tuberculosis-endemic country: diagnostic efficacy in 674 patients. Cancer. 2007;109:1068–77.
Kim BT, Lee KS, Shim SS, Choi JY, Kwon OJ, Kim H, et al. Stage T1 non-small cell lung cancer: preoperative mediastinal nodal staging with integrated FDG PET/CT–a prospective study. Radiology. 2006;241:501–9.
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54.
Chen YH, Chu SC, Wang LY, Wang TF, Lue KH, Lin CB, et al. Prognostic value of combing primary tumor and nodal glycolytic-volumetric parameters of 18F-FDG PET in patients with non-small cell lung cancer and regional lymph node metastasis. Diagnostics (Basel). 2021;11:1065.
Traverso A, Wee L, Dekker A, Gillies R. Repeatability and reproducibility of radiomic features: a systematic review. Int J Radiat Oncol Biol Phys. 2018;102:1143–58.
Desseroit MC, Tixier F, Weber WA, Siegel BA, Cheze Le Rest C, Visvikis D, et al. Reliability of PET/CT shape and heterogeneity features in functional and morphologic components of non-small cell lung cancer tumors: a repeatability analysis in a prospective multicenter cohort. J Nucl Med. 2017;58:406–11.
Oliver JA, Budzevich M, Zhang GG, Dilling TJ, Latifi K, Moros EG. Variability of image features computed from conventional and respiratory-gated PET/CT images of lung cancer. Transl Oncol. 2015;8:524–34.
Xu H, Lv W, Zhang H, Ma J, Zhao P, Lu L. Evaluation and optimization of radiomics features stability to respiratory motion in 18F-FDG 3D PET imaging. Med Phys. 2021;48:5165–78.
van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77:e104–7.
Zwanenburg A, Vallieres M, Abdalah MA, Aerts HJ, Andrearczyk V, Apte A, et al. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology. 2020;295:328–38.
Polley MC, Dignam JJ. Statistical considerations in the evaluation of continuous biomarkers. J Nucl Med. 2021;62:605–11.
Zwanenburg A. Radiomics in nuclear medicine: robustness, reproducibility, standardization, and how to avoid data analysis traps and replication crisis. Eur J Nucl Med Mol Imaging. 2019;46:2638–55.
Okour M, Jacobson PA, Israni A, Brundage RC. Comparative evaluation of median versus Youden index dichotomization methods: exposure-response analysis of mycophenolic acid and acyl-glucuronide metabolite. Eur J Drug Metab Pharmacokinet. 2019;44:629–38.
Aragaki M, Kato T, Fujiwara-Kuroda A, Hida Y, Kaga K, Wakasa S. Preoperative identification of clinicopathological prognostic factors for relapse-free survival in clinical N1 non-small cell lung cancer: a retrospective single center-based study. J Cardiothorac Surg. 2020;15:229.
Turajlic S, Swanton C. Metastasis as an evolutionary process. Science. 2016;352:169–75.
Caswell DR, Chuang CH, Yang D, Chiou SH, Cheemalavagu S, Kim-Kiselak C, et al. Obligate progression precedes lung adenocarcinoma dissemination. Cancer Discov. 2014;4:781–9.
Moon SH, Kim J, Joung JG, Cha H, Park WY, Ahn JS, et al. Correlations between metabolic texture features, genetic heterogeneity, and mutation burden in patients with lung cancer. Eur J Nucl Med Mol Imaging. 2019;46:446–54.
Kim G, Kim J, Cha H, Park WY, Ahn JS, Ahn MJ, et al. Metabolic radiogenomics in lung cancer: associations between FDG PET image features and oncogenic signaling pathway alterations. Sci Rep. 2020;10:13231.
Sellitto A, Pecoraro G, Giurato G, Nassa G, Rizzo F, Saggese P, et al. Regulation of metabolic reprogramming by long non-coding RNAs in cancer. Cancers (Basel). 2021;13:3485.
Pijl JP, Nienhuis PH, Kwee TC, Glaudemans AW, Slart RH, Gormsen LC. Limitations and pitfalls of FDG-PET/CT in infection and inflammation. Semin Nucl Med. 2021. https://doi.org/10.1053/j.semnuclmed.2021.06.008.
Chiu LC, Lin SM, Lo YL, Kuo SC, Yang CT, Hsu PC. Immunotherapy and vaccination in surgically resectable non-small cell lung cancer (NSCLC). Vaccines (Basel). 2021;9:689.
Shukla N, Hanna N. Neoadjuvant and adjuvant immunotherapy in early-stage non-small cell lung cancer. Lung Cancer (Auckl). 2021;12:51–60.
Zhong WZ, Wang Q, Mao WM, Xu ST, Wu L, Wei YC, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1–N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 phase III trial. J Clin Oncol. 2021;39:713–22.
Provencio M, Nadal E, Insa A, Garcia-Campelo MR, Casal-Rubio J, Domine M, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1413–22.
van Velden FH, Kramer GM, Frings V, Nissen IA, Mulder ER, de Langen AJ, et al. Repeatability of radiomic features in non-small-cell lung cancer [(18)F]FDG-PET/CT studies: impact of reconstruction and delineation. Mol Imaging Biol. 2016;18:788–95.
Konert T, Everitt S, La Fontaine MD, van de Kamer JB, MacManus MP, Vogel WV, et al. Robust, independent and relevant prognostic 18F-fluorodeoxyglucose positron emission tomography radiomics features in non-small cell lung cancer: are there any? PLoS ONE. 2020;15:e0228793.
Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007;48:932–45.
Acknowledgements
We would like to express our deepest and sincere appreciation to staff from the Cancer Center of Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation for their kind assistance in retrieving the data of patients with lung cancer.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no potential conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The requirement of informed consent for this study was waived due to its retrospective nature.
Informed consent
The requirement of informed consent for this study was waived due to its retrospective nature.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lue, KH., Chu, SC., Wang, LY. et al. Tumor glycolytic heterogeneity improves detection of regional nodal metastasis in patients with lung adenocarcinoma. Ann Nucl Med 36, 256–266 (2022). https://doi.org/10.1007/s12149-021-01698-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-021-01698-1