Abstract
Objective
To evaluate the reproducibility of cerebral adenosine A2A receptor (A2AR) quantification using [11C]preladenant ([11C]PLN) and PET in a test–retest study.
Methods
Eight healthy male volunteers were enrolled. Dynamic 90 min PET scans were performed twice at the same time of the day to avoid the effect of diurnal variation. Subjects refrained from caffeine from 12 h prior to scanning, and serum caffeine was measured before radioligand injection. Arterial blood was sampled repeatedly during scanning and the fraction of the parent compound in plasma was determined. Total distribution volume (VT) was estimated using 1- and 2-tissue compartment models (1-TCM and 2-TCM, respectively) and Logan graphical analysis (Logan plot) (t* = 30 min). Plasma-free fraction (fP) of [11C]PLN was measured and used for correction of VT values. Distribution volume ratio (DVR) was calculated from VT of target and reference regions and obtained by noninvasive Logan graphical reference tissue model (LGAR) (t* = 30 min). Absolute test–retest variability (aTRV), and intra-class correlation coefficient (ICC) of VT and DVR were calculated as indexes of repeatability. Correlation between DVR and serum concentration of caffeine (a nonselective A2AR blocker) was analyzed by Pearson’s correlation analysis.
Results
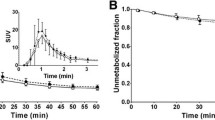
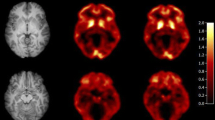
Regional time–activity curves were well described by 2-TCM models. Estimation of VT by 2-TCM produced some erroneous values; therefore, the more robust Logan plot was selected as the appropriate model. Global mean aTRV was 20% for VT and 14% for VT/fP (ICC, 0.72 for VT and 0.87 for VT/fP). Global mean aTRV of DVR was 13% for Logan plot and 10% for LGAR (ICC, 0.70 for Logan plot and 0.81 for LGAR). DVR estimates using LGAR and Logan plot were in good agreement (r2 = 0.96). Coefficients of variation for VT, VT/fP, DVR (Logan plot), and DVR (LGAR) were 47%, 47%, 27%, and 18%, respectively. Despite low serum caffeine levels, significant concentration-dependent effects on [11C]PLN binding to target regions were observed (p < 0.01).
Conclusions
In this study, moderate test–retest reproducibility and large inter-subject differences were observed with [11C]PLN PET, possibly attributable to competition by baseline amount of caffeine. Analysis of plasma caffeine concentration is recommended during [11C]PLN PET studies.
Trial registration
UMIN000030040.





Similar content being viewed by others
Availability of data and materials
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
van Waarde A, Dierckx RAJO, Zhou X, Khanapur S, Tsukada H, Ishiwata K, et al. Potential therapeutic application of adenosine A2A receptor ligands and opportunities for A2A receptor imaging. Med Res Rev. 2018;38:5–56.
Paton DM. Istradefylline: adenosine A2A receptor antagonist to reduce “off” time in Parkinson’s disease. Drugs Today (Barc). 2020;56:125–34.
Naganawa M, Kimura Y, Mishina M, Manabe Y, Chihara K, Oda K, et al. Quantification of adenosine A2A receptors in the human brain using [11C]TMSX and positron emission tomography. Eur J Nucl Med Mol Imaging. 2007;34:679–87.
Brooks DJ, Doder M, Osman S, Luthra SK, Hirani E, Hume S, et al. Positron emission tomography analysis of [11C]KW-6002 binding to human and rat adenosine A2A receptors in the brain. Synapse. 2008;62:671–81.
Ramlackhansingh AF, Bose SK, Ahmed I, Turkheimer FE, Pavese N, Brooks DJ. Adenosine 2A receptor availability in dyskinetic and nondyskinetic patients with Parkinson disease. Neurology. 2011;76:1811–6.
Sakata M, Ishibashi K, Imai M, Wagatsuma K, Ishii K, Zhou X, et al. Initial evaluation of an adenosine A2A receptor ligand, 11C-preladenant, in healthy human subjects. J Nucl Med. 2017;58:1464–70.
Barret O, Hannestad J, Vala C, Alagille D, Tavares A, Laruelle M, et al. Characterization in humans of 18F-MNI-444, a PET radiotracer for brain adenosine 2A receptors. J Nucl Med. 2015;56:586–91.
Ishibashi K, Miura Y, Wagatsuma K, Toyohara J, Ishiwata K, Ishii K. Occupancy of adenosine A2A receptors by istradefylline in patients with Parkinson’s disease using 11C-preladenant PET. Neuropharmacology. 2018;143:106–12.
Ishibashi K, Miura Y, Wagatsuma K, Toyohara J, Ishiwata K, Ishii K. Adenosine A2A receptor occupancy by long-term istradefylline administration in Parkinson’s disease. Mov Disord. 2021;38:268–9.
Wagatsuma K, Miwa K, Sakata M, Oda K, Ono H, Kameyama M, et al. Comparison between new-generation SiPM-based and conventional PMT-based TOF-PET/CT. Phys Med. 2017;42:203–10.
Toyohara J, Yamamoto H, Tago T. Searching for diagnostic properties of novel fluorine-18-labeled D-allose. Ann Nucl Med. 2019;33:855–65.
Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurement applied to [N-11C-methyl]-(–)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–7.
Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–40.
Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rather reliability. Psychol Bull. 1979;86:420–8.
Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression—a new method based on robust nonlinear regression and false discovery rate. BMC Bioinformatics. 2006;7:123.
Zhou X, Khanapur S, de Jong JR, Willemsen ATM, Dierckx RAJO, Elsinga PH, et al. In vivo evaluation of [11C]preladenant positron emission tomography for quantification of adenosine A2A receptors in the rat brain. J Cereb Blood Flow Metab. 2017;37:577–89.
Eckelman WC, Kilbourn MR, Mathis CA. Specific to nonspecific binding in radiopharmaceutical studies: it’s not so simple as it seems! Nucl Med Biol. 2009;36:235–7.
Zhou X, Boellaard R, Ishiwata K, Sakata M, Dierckx RAJO, de Joung JR, et al. In vivo evaluation of 11C-preladenant for PET imaging of adenosine A2A receptors in conscious monkey. J Nucl Med. 2017;58:762–7.
Ottoy J, De Picker L, Verhaeghe J, Deleye S, Wyffels L, Kosten L, et al. 18F-PBR111 PET imaging in healthy controls and schizophrenia: test–retest reproducibility and quantification of neuroinflammation. J Nucl Med. 2018;59:1267–74.
Mase H, Tanaka A, Sasou T, Nozaki A, Asai S, Miyachi H. Simultaneous measurement of concentrations of caffeine and ibuprofen in blood by LC-MS/MS and pharmacokinetic analysis after oral intake of canned coffee. Jpn J Med Technol. 2015;64:413–20.
Jacobson KA, Gao Z-G, Matricon P, Eddy MT, Carisson J. Adenosine A2A receptor antagonists: from caffeine to selective non-xanthines. Br J Pharmacol. 2020. https://doi.org/10.1111/bph.15103.
White JR Jr, Padowski JM, Zhong Y, Chen G, Luo S, Lazarus P, et al. Pharmacokinetic analysis and comparison of caffeine administered rapidly or slowly in coffee chilled or hot versus chilled energy drink in healthy young adults. Clin Toxicol (Phila). 2016;54:308–12.
Caffeine for the Sustainment of Mental Task, Performance: Formulations for Military Operations. Committee on Military Nutrition Research, Food, and Nutrition Board. National Academy of Sciences; [cited 2015 Oct]. Available from: https://iom.nationalacademies.org/Reports/2001/Caffeine-for-the-Sustainment-of-Mental-Task-Performance-Formulations-for-Military-Operations.aspx. Accessed 8 June 2021.
Elmenhorst D, Meyer PT, Matusch A, Winz OH, Bauer A. Caffeine occupancy of human cerebral A1 adenosine receptors: in vivo quantification with 18F-CPFPX and PET. J Nucl Med. 2012;53:1723–9.
Acknowledgements
We thank Mr. Masanari Sakai and Mr. Kosuke Nishino for technical support with cyclotron operation and radiosynthesis, Ms. Kimiko Yokoyama for care of the subjects during PET scanning, and Ms. Airin Onishi for coordination of the clinical study.
Funding
This work was supported in part by Grants-in-Aid for Scientific Research (B) No. 16H05396 (K.I.) and (C) No. 21K07663 (J.T.) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All procedures performed in studies involving human participants were approved by the Ethics Committee of the Tokyo Metropolitan Institute of Gerontology (H28-46), and in accordance with the principles of the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
Written informed consent was obtained from all individual participants prior to their inclusion in the study.
Consent to publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Toyohara, J., Sakata, M., Wagatsuma, K. et al. Test–retest reproducibility of cerebral adenosine A2A receptor quantification using [11C]preladenant. Ann Nucl Med 36, 15–23 (2022). https://doi.org/10.1007/s12149-021-01678-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-021-01678-5