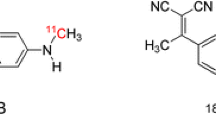
Abstract
Alzheimer’s disease (AD) is on the rise all over the world, and brings with it great challenges to medical care and heavy burdens to family and society. Accurate diagnosis and differential diagnosis are of great importance. Tau positron emission tomography (PET) might offer novel insights and be of great assistance in monitoring disease progression and supporting the differential diagnosis. 18F-AV-1451, as the first Tau PET imaging agent approved by the Food and Drug Administration (FDA), has been of great potential in clinical trials. Here, we reviewed the synthesis and characteristics of 18F-AV-1451 and its role in monitoring AD progression and supporting the differential diagnosis.

Similar content being viewed by others
References
Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179(2):312–39.
Hodson R. Alzheimer’s disease. Nature. 2018;559(7715):S1.
Giacobini E, Gold G. Alzheimer disease therapy—moving from amyloid-beta to tau. Nat Rev Neurol. 2013;9(12):677–86.
Tosun D, Landau S, Aisen PS, Petersen RC, Mintun M, Jagust W, et al. Association between tau deposition and antecedent amyloid-β accumulation rates in normal and early symptomatic individuals. Brain. 2017;140(5):1499–512.
Mattsson N, Smith R, Strandberg O, Palmqvist S, Schöll M, Insel PS, et al. Comparing 18F-AV-1451 with CSF t-tau and p-tau for diagnosis of Alzheimer disease. Neurology. 2018;90(5):e388–95.
Xia CF, Arteaga J, Chen G, Gangadharmath U, Gomez LF, Kasi D, et al. [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement. 2013;9(6):666–76.
Marquié M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78(5):787–800.
Salabert AS, Fontan C, Fonta C, Alonso M, Loukh N, Delisle MB, et al. Radiosynthesis of [F]AV1451 in pharmaceutical conditions and its biological characteristics. Appl Radiat Isot. 2017;128:101–7.
Golla SSV, Timmers T, Ossenkoppele R, Groot C, Verfaillie S, Scheltens P, et al. Quantification of tau load using [F]AV1451 PET. Mol Imaging Biol. 2017;19(6):963–71.
Holt DP, Ravert HT, Dannals RF. Synthesis and quality control of [(18) F]T807 for tau PET imaging. J Labelled Comp Radiopharm. 2016;59(10):411–5.
Chien DT, Szardenings AK, Bahri S, Walsh JC, Mu F, Xia C, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis. 2013;34(2):457–68.
Shoup TM, Yokell DL, Rice PA, Jackson RN, Livni E, ohnson KA, et al. A concise radiosynthesis of the tau radiopharmaceutical, [(18) F]T807. J Labelled Comp Radiopharm. 2013;56(14):736–40.
Liang SH, Yokell DL, Normandin MD, Rice PA, Jackson RN, Shoup TM, et al. First human use of a radiopharmaceutical prepared by continuous-flow microfluidic radiofluorination: proof of concept with the tau imaging agent [18F]T807. Mol Imaging. 2014;13(8):1–5.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59.
Knopman DS. Alzheimer disease: preclinical Alzheimer disease—the new frontier. Nat Rev Neurol. 2016;12(11):620–1.
Hoenig MC, Bischof GN, Seemiller J, Hammes J, Kukolja J, Onur ÖA, et al. Networks of tau distribution in Alzheimer’s disease. Brain. 2018;141(2):568–81.
Dronse J, Fliessbach K, Bischof GN, von Reutern B, Faber J, Hammes J, et al. In vivo patterns of tau pathology, amyloid-β burden, and neuronal dysfunction in clinical variants of Alzheimer’s disease. J Alzheimers Dis. 2017;55(2):465–71.
Schwarz AJ, Yu P, Miller BB, Shcherbinin S, Dickson J, Navitsky M, et al. Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain. 2016;139(5):1539–50.
Wang YT, Edison P. Tau imaging in neurodegenerative diseases using positron emission tomography. Curr Neurol Neurosci Rep. 2019;19(7):45.
Das SR, Xie L, Wisse LEM, Ittyerah R, Tustison NJ, Dickerson BC, et al. Longitudinal and cross-sectional structural magnetic resonance imaging correlates of AV-1451 uptake. Neurobiol Aging. 2018;66:49–58.
Smith R, Wibom M, Pawlik D, Englund E, Hansson O. Correlation of in vivo [18F]flortaucipir with postmortem Alzheimer disease tau pathology. JAMA Neurol. 2019;76(3):310–7.
Xie L, Das SR, Wisse LEM, Ittyerah R, Yushkevich PA, Wolk DA. Early tau burden correlates with higher rate of atrophy in transentorhinal cortex. J Alzheimers Dis. 2018;62(1):85–92.
Gordon BA, Friedrichsen K, Brier M, Blazey T, Su Y, Christensen J, et al. The relationship between cerebrospinal fluid markers of Alzheimer pathology and positron emission tomography tau imaging. Brain. 2016;139(8):2249–60.
Ossenkoppele R, Smith R, Ohlsson T, Strandberg O, Mattsson N, Insel PS, et al. Associations between tau, Aβ, and cortical thickness with cognition in Alzheimer disease. Neurology. 2019;92(6):e601–12.
Schöll M, Ossenkoppele R, Strandberg O, Palmqvist S, Jögi J, Ohlsson T, et al. Distinct 18F-AV-1451 tau PET retention patterns in early- and late-onset Alzheimer’s disease. Brain. 2017;140(9):2286–94.
Pontecorvo MJ, Devous MD, Kennedy I, Navitsky M, Lu M, Galante N, et al. A multicentre longitudinal study of flortaucipir (18F) in normal ageing, mild cognitive impairment and Alzheimer’s disease dementia. Brain. 2019;142(6):1723–35.
Harrison TM, La Joie R. Longitudinal tau accumulation and atrophy in aging and Alzheimer disease. Ann Neurol. 2019;85(2):229–40.
Cho H, Choi JY, Lee HS, Lee JH, Ryu YH, Lee MS, et al. Progressive tau accumulation in Alzheimer disease: 2 year follow-up study. J Nucl Med. 2019;60(11):1611–21.
Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, et al. Addendum: the antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2017;546(7659):564.
Vaillancourt DE. Aducanumab reduces Abeta plaques in Alzheimer’s disease. Mov Disord: Off J Mov Disord Soc. 2016;31(11):1631.
Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–6.
Vaz M, Silvestre S. Alzheimer’s disease: recent treatment strategies. Eur J Pharmacol. 2020;887(173554):1–13.
Schneider L. A resurrection of aducanumab for Alzheimer’s disease. The Lancet Neurol. 2020;19(2):111–2.
Biogen. Biogen plans regulatory filing for Aducanumab in Alzheimer’s disease based on new analysis of larger dataset from phase 3 studies. Available from: https://investors.biogen.com/news-releases/news-release-details/biogen-plans-regulatory-filing-aducanumab-alzheimers-disease.
Biogen. Biogen completes submission of biologics license application to Fda for Aducanumab as a treatment for Alzheimer’s disease. Available from: https://investors.biogen.com/news-releases/news-release-details/biogen-completes-submission-biologics-license-application-fda.
Uddin MS, Kabir MT, Rahman MS, Behl T, Jeandet P, Ashraf GM, et al. Revisiting the amyloid cascade hypothesis: from anti-abeta therapeutics to auspicious new ways for Alzheimer’s disease. Int J Mol Sci. 2020;21(16):1–34.
Congdon EE, Sigurdsson EM. Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2018;14(7):399–415.
Imbimbo BP, Lozupone M, Watling M, Panza F. Discontinued disease-modifying therapies for Alzheimer’s disease: status and future perspectives. Expert Opin Investig Drugs. 2020;29(9):919–33.
Cummings J, Blennow K, Johnson K, Keeley M, Bateman RJ, Molinuevo JL, et al. Anti-tau trials for Alzheimer’s disease: a report from the EU/US/CTAD task force. J Prev Alzheimers Dis. 2019;6(3):157–63.
Arakhamia T, Lee CE, Carlomagno Y, Duong DM, Kundinger SR, Wang K, et al. Posttranslational modifications mediate the structural diversity of tauopathy strains. Cell. 2020;180(4):633-44.e12.
Zhang W, Tarutani A, Newell KL, et al. Novel tau filament fold in corticobasal degeneration. Nature. 2020;580(7802):283–7.
Falcon B, Zhang W, Murzin AG, et al. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature. 2019;568(7752):420–3.
Kovacs GG. Invited review: neuropathology of tauopathies: principles and practice. Neuropathol Appl Neurobiol. 2015;41(1):3–23.
Falcon B, Zhang W, Murzin AG, Murshudov G, Garringer HJ, Vidal R, et al. Structures of filaments from Pick’s disease reveal a novel tau protein fold. Nature. 2018;561(7721):137–40.
Dujardin S, Commins C, Lathuiliere A, Beerepoot P, Fernandes AR, Kamath TV, et al. Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat Med. 2020. https://doi.org/10.1038/s41591-020-0938-9.
Dujardin S, Bégard S, Caillierez R, Lachaud C, Carrier S, Lieger S, et al. Different tau species lead to heterogeneous tau pathology propagation and misfolding. Acta Neuropathol Commun. 2018;6(1):132.
Smith R, Schöll M, Londos E, Ohlsson T, Hansson O. 18F-AV-1451 in Parkinson’s disease with and without dementia and in dementia with Lewy bodies. Sci Rep. 2018;8(1):4717.
Lee SH, Cho H, Choi JY, Lee JH, Ryu YH, Lee MS, et al. Distinct patterns of amyloid-dependent tau accumulation in Lewy body diseases. Mov Disord. 2018;33(2):262–72.
Schonhaut DR, McMillan CT, Spina S, Dickerson BC, Siderowf A, Devous MD Sr, et al. (18) F-flortaucipir tau positron emission tomography distinguishes established progressive supranuclear palsy from controls and Parkinson disease: a multicenter study. Ann Neurol. 2017;82(4):622–34.
Passamonti L, Vázquez Rodríguez P, Hong YT, Allinson KS, Williamson D, Borchert RJ, et al. 18F-AV-1451 positron emission tomography in Alzheimer’s disease and progressive supranuclear palsy. Brain. 2017;140(3):781–91.
Whitwell JL, Lowe VJ, Tosakulwong N, Weigand SD, Senjem ML, Schwarz CG, et al. [(18) F]AV-1451 tau positron emission tomography in progressive supranuclear palsy. Mov Disord. 2017;32(1):124–33.
Smith R, Schöll M, Widner H, van Westen D, Svenningsson P, Hägerström D, et al. In vivo retention of 18F-AV-1451 in corticobasal syndrome. Neurology. 2017;89(8):845–53.
La Joie R, Bejanin A, Fagan AM, Ayakta N, Baker SL, Bourakova V, et al. Associations between [(18)F]AV1451 tau PET and CSF measures of tau pathology in a clinical sample. Neurology. 2018;90(4):e282–90.
Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, et al. Alzheimer’s disease. The Lancet. 2016;388(10043):505–17.
Laruelle M, Slifstein M, Huang Y. Relationships between radiotracer properties and image quality in molecular imaging of the brain with positron emission tomography. Mol Imaging Biol. 2003;5(6):363–75.
Pike VW. PET radiotracers: crossing the blood-brain barrier and surviving metabolism. Trends Pharmacol Sci. 2009;30(8):431–40.
Jang YK, Lyoo CH, Park S, Oh SJ, Cho H, Oh M, et al. Head to head comparison of [(18)F] AV-1451 and [(18)F] THK5351 for tau imaging in Alzheimer’s disease and frontotemporal dementia. Eur J Nucl Med Mol Imaging. 2018;45(3):432–42.
Vemuri P, Lowe VJ, Knopman DS, Senjem ML, Kemp BJ, Schwarz CG, et al. Tau-PET uptake: regional variation in average SUVR and impact of amyloid deposition. Alzheimers Dement (Amst). 2016;6:21–30.
Chen J, Li Y, Pirraglia E, Okamura N, Rusinek H, de Leon MJ. Quantitative evaluation of tau PET tracers (18)F-THK5351 and (18)F-AV-1451 in Alzheimer’s disease with standardized uptake value peak-alignment (SUVP) normalization. Eur J Nucl Med Mol Imaging. 2018;45(9):1596–604.
Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun. 2016;4:58.
Marquié M, Verwer EE, Meltzer AC, Kim SJW, Agüero C, Gonzalez J, et al. Lessons learned about [F-18]-AV-1451 off-target binding from an autopsy-confirmed Parkinson’s case. Acta Neuropathol Commun. 2017;5(1):75.
Hansen AK, Knudsen K, Lillethorup TP, Landau AM, Parbo P, Fedorova T, et al. In vivo imaging of neuromelanin in Parkinson’s disease using 18F-AV-1451 PET. Brain. 2016;139:2039–49.
Wolters EE, Golla SSV, Timmers T, Ossenkoppele R, van der Weijden CWJ, Scheltens P, et al. A novel partial volume correction method for accurate quantification of [F] flortaucipir in the hippocampus. EJNMMI Res. 2018;8(1):79.
Lee CM, Jacobs HIL, Marquié M, Becker JA, Andrea NV, Jin DS, et al. 18F-Flortaucipir binding in choroid plexus: related to race and hippocampus signal. J Alzheimers Dis. 2018;62(4):1691–702.
Wolters EE, Ossenkoppele R, Golla SS, Verfaillie SC, Timmers T, Visser D, et al. Hippocampal [18F]flortaucipir BPND corrected for possible spill-in of the choroid plexus retains strong clinico-pathological relationships. Neuroimage Clin. 2020;25:102113.
Drake LR, Pham JM, Desmond TJ, Mossine AV, Lee SJ, Kilbourn MR, et al. Identification of AV-1451 as a weak, nonselective inhibitor of monoamine oxidase. ACS Chem Neurosci. 2019;10(8):3839–46.
Murugan NA, Chiotis K, Rodriguez-Vieitez E, Lemoine L, Ågren H, Nordberg A. Cross-interaction of tau PET tracers with monoamine oxidase B: evidence from in silico modelling and in vivo imaging. Eur J Nucl Med Mol Imaging. 2019;46(6):1369–82.
Hansen AK, Brooks DJ, Borghammer P. MAO-B Inhibitors do not block in vivo flortaucipir([F]-AV-1451) binding. Mol Imaging Biol. 2018;20(3):356–60.
Ng KP, Pascoal TA, Mathotaarachchi S, Therriault J, Kang MS, Shin M, et al. Monoamine oxidase B inhibitor, selegiline, reduces F-THK5351 uptake in the human brain. Alzheimers Res Ther. 2017;9(1):25.
Vermeiren C, Mercier J, Viot D, Mairet-Coello G, Hannestad J, Courade JP, et al. T807, a reported selective tau tracer, binds with nanomolar affinity to monoamine oxidase A. Alzheimer Demen. 2015;11(7):283.
Lemoine L, Gillberg PG, Svedberg M, Stepanov V, Jia Z, Huang J, et al. Comparative binding properties of the tau PET tracers THK5117, THK5351, PBB3, and T807 in postmortem Alzheimer brains. Alzheimers Res Ther. 2017;9(1):96.
Leuzy A, Smith R, Ossenkoppele R, Santillo A, Borroni E, Klein G, et al. Diagnostic performance of RO948 F 18 tau positron emission tomography in the differentiation of Alzheimer disease from other neurodegenerative disorders. JAMA Neurol. 2020. https://doi.org/10.1001/jamaneurol.2020.0989.
Honer M, Gobbi L, Knust H, Kuwabara H, Muri D, Koerner M, et al. Preclinical evaluation of F-RO6958948, C-RO6931643, and C-RO6924963 as novel PET radiotracers for imaging tau aggregates in Alzheimer disease. J Nucl Med. 2018;59(4):675–81.
Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25(1):59–70.
Funding
This study was funded by the National Natural Science Foundation of China (81971125 and 81771868) and Natural Science Foundation of Liaoning Province (2017010083-301).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, W., Xu, S., Yu, H. et al. Radioactive synthesis of tau PET imaging agent 18F-AV-1451 and its role in monitoring the progression of Alzheimer’s disease and supporting differential diagnosis. Ann Nucl Med 35, 139–147 (2021). https://doi.org/10.1007/s12149-020-01566-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-020-01566-4