Abstract
Objective
Statistical image analysis of brain SPECT images has improved diagnostic accuracy for brain disorders. However, the results of statistical analysis vary depending on the institution even when they use a common normal database (NDB), due to different intrinsic spatial resolutions or correction methods. The present study aimed to evaluate the correction of spatial resolution differences between equipment and examine the differences in skull bone attenuation to construct a common NDB for use in multicenter settings.
Methods
The proposed acquisition and processing protocols were those routinely used at each participating center with additional triple energy window (TEW) scatter correction (SC) and computed tomography (CT) based attenuation correction (CTAC). A multicenter phantom study was conducted on six imaging systems in five centers, with either single photon emission computed tomography (SPECT) or SPECT/CT, and two brain phantoms. The gray/white matter I-123 activity ratio in the brain phantoms was 4, and they were enclosed in either an artificial adult male skull, 1300 Hounsfield units (HU), a female skull, 850 HU, or an acrylic cover. The cut-off frequency of the Butterworth filters was adjusted so that the spatial resolution was unified to a 17.9 mm full width at half maximum (FWHM), that of the lowest resolution system. The gray-to-white matter count ratios were measured from SPECT images and compared with the actual activity ratio. In addition, mean, standard deviation and coefficient of variation images were calculated after normalization and anatomical standardization to evaluate the variability of the NDB.
Results
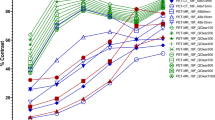
The gray-to-white matter count ratio error without SC and attenuation correction (AC) was significantly larger for higher bone densities (p < 0.05). The count ratio error with TEW and CTAC was approximately 5% regardless of bone density. After adjustment of the spatial resolution in the SPECT images, the variability of the NDB decreased and was comparable to that of the NDB without correction.
Conclusion
The proposed protocol showed potential for constructing an appropriate common NDB from SPECT images with SC, AC and spatial resolution compensation.






Similar content being viewed by others
References
McArthur C, Jampana R, Patterson J, Hadley D. Applications of cerebral SPECT. Clin Radiol. 2011;66:651 – 61.
Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377:1019–31.
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89:88–100.
Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734 – 46.
Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995;36:1238–48.
Friston KJ. Analyzing brain images: Principles and overview. In: Frackowiak RSJ, Friston KJ, Frith CD et al, editors. Human Brain Function. San Diego, CA: Academic Press; 1997. pp. 25–41.
Iida H, Narita Y, Kado H, Kashikura A, Sugawara S, Shoji Y, et al. Effects of scatter and attenuation correction on quantitative assessment of regional cerebral blood flow with SPECT. J Nucl Med. 1998;39:181–9.
Shimada H, Otake H, Higuchi T, Arisaka Y, Oriuchi N, Endo K. Normal database (NDB) of 123I-IMP brain perfusion SPECT examination is affected by statistical image analysis in the presence or absence of scatter correction and attenuation correction. Kaku Igaku. 2012;49:341–9. (ArticleJapanese).
Onishi H, Matsutake Y, Matsumoto N, Kai Y, Amijima H. Effect of prefiltering cutoff frequency and scatter and attenuation corrections during normal database creation for statistical imaging analysis of the brain. J Nucl Med Technol. 2011;39:231–6.
Chang LT. A method for attenuation correction in radionuclide computed tomography. IEEE Trans Nucl Sci. 1977;25:638–43.
Stodilka RZ, Kemp BJ, Prato FS, Nicholson RL. Importance of bone attenuation in brain SPECT quantification. J Nucl Med. 1998;39:190–7.
Hayashi M, Deguchi J, Utsunomiya K, Yamada M, Komori T, Takeuchi M, et al. Comparison of methods of attenuation and scatter correction in brain perfusion SPECT. J Nucl Med Technol. 2005;33:224–9.
Farid K, Habert MO, Martineau A, Caillat-Vigneron N, Sibon I. CT nonuniform attenuation and TEW scatter corrections in brain Tc-99m ECD SPECT. Clin Nucl Med. 2011;36:665–8.
Ishii K, Hanaoka K, Okada M, Kumano S, Komeya Y, Tsuchiya N, Hosono M, Murakami T. Impact of CT attenuation correction by SPECT/CT in brain perfusion images. Ann Nucl Med. 2012;26:241–7.
Ichihara T, Ogawa K, Motomura N, Kubo A, Hashimoto S. Compton scatter compensation using the triple-energy window method for single- and dual-isotope SPECT. J Nucl Med. 1993;34:2216–21.
Meikle SR, Hutton BF, Bailey DL. A transmission dependent method for scatter correction in SPECT. J Nucl Med. 1994;35:360–7.
Beekman FJ, Kamphuis C, Frey EC. Scatter compensation methods in 3D iterative SPECT reconstruction: a simulation study. Phys Med Biol. 1997;42:1619–32.
Ljungberg M, King A, Hademensos G, Strand E. Comparison of four scatter correction methods using Monte Carlo simulated source distributions. J Nucl Med. 1994;35:143–51.
Minoshima S, Koeppe RA, Frey KA, Kuhl DE. Anatomic standardization: linear scaling and nonlinear warping of functional brain images. J Nucl Med. 1994;35:1528–37.
Acknowledgements
This work was performed by the working group of Japanese Society of Nuclear Medicine (JSNM) in 2013–2015 and was supported partly by JSNM. No potential conflicts of interest were disclosed.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Inui, Y., Ichihara, T., Uno, M. et al. CT-based attenuation correction and resolution compensation for I-123 IMP brain SPECT normal database: a multicenter phantom study. Ann Nucl Med 32, 311–318 (2018). https://doi.org/10.1007/s12149-018-1248-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-018-1248-x