Abstract
Objective
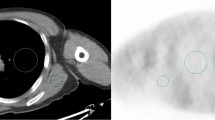
On 18F-fluoro-2-deoxy-d-glucose (18F-FDG) positron emission tomography (PET), signal-to-noise ratio in the liver (SNRliver) is used as a metric to assess image quality. However, some regions-of-interest (ROIs) are used when measuring the SNRliver. The purpose of this study is to examine the different ROIs and volumes of interest (VOIs) to obtain a reproducible SNRliver.
Methods
This study included 108 patients who underwent 18F-FDG-PET/CT scans for the purpose of cancer screening. We examined four different ROIs and VOIs; a 3-cm-diameter and a 4-cm-diameter circular ROI and a 3-cm-diameter and a 4-cm-diameter spherical VOI on the right lobe of the patients’ livers. The average of SUV (SUVmean), standard deviation (SD) of SUV (SUVSD), SNRliver and SD of the SNRliver obtained using ROIs and VOIs were then compared.
Results
Although the SUVmean was not different among the ROIs and VOIs, the SUVSD was small with a 3-cm-diameter ROI. The largest SUVSD was obtained with a 4-cm-diameter spherical VOI. The SNRliver and the SD of the SNRliver with a 4-cm-diameter spherical VOI were the smallest, while those with a 3-cm-diameter circular ROI were the largest. These results suggest that a small ROI may be placed on a relatively homogeneous region not representing whole liver unintentionally.
Conclusion
The SNRliver varied according to the shape and size of ROIs or VOIs. A 4-cm-diameter spherical VOI is recommended to obtain stable and reproducible SNRliver.





Similar content being viewed by others
References
Lindell RM, Hartman TE, Swensen SJ, Jett JR, Midthun DE, Nathan MA, et al. Lung cancer screening experience: a retrospective review of PET in 22 non-small cell lung carcinomas detected on screening chest CT in a high-risk population. Am J Roentgenol. 2005;185:126–31.
Cronin P, Dwamena BA, Kelly AM, Carlos RC. Solitary pulmonary nodules: meta-analytic comparison of cross-sectional imaging modalities for diagnosis of malignancy. Radiology. 2008;246:772–82.
Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasu K, Uno K. The size of metastatic foci and lymph nodes yielding false-negative and false–positive lymph node staging with positron emission tomography in patients with lung cancer. J Thorac Cardiovasc Surg. 2004;127:1087–92.
Lee JH, Rosen EL, Mankoff DA. The role of radiotracer imaging in the diagnosis and management of patients with breast cancer: part 1—overview, detection, and staging. J Nucl Med. 2009;50:569–81.
Lee JH, Rosen EL, Mankoff DA. The role of radiotracer imaging in the diagnosis and management of patients with breast cancer: part 2—response to therapy, other indications, and future directions. J Nucl Med. 2009;50:738–48.
Kato H, Miyazaki T, Nakajima M, Takita J, Kimura H, Faried A, et al. The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer. 2005;103:148–56.
van Vliet EPM, Heijenbrok-Kal MH, Hunink MGM, Kulpers EJ, Siersema PD. Staging investigations for oesophageal cancer: a meta analysis. Br J Cancer. 2008;98:547–57.
Israel O, Yefremov N, Bar-Shalom R, Kagana O, Frenkel A, Keidar Z, et al. PET/CT detection of unexpected gastrointestinal foci of 18F-FDG uptake: incidence, localization patterns, and clinical significance. J Nucl Med. 2005;46:758–62.
Bares R, Klever P, Hauptmann S, Hellwig D, Fass J, Cremerius U, et al. F-18 fluorodeoxyglucose PET in vivo evaluation of pancreatic glucose metabolism for detection of pancreatic cancer. Radiology. 1994;192:79–86.
Bang S, Chung HW, Park SW, Chung JB, Yun M, Lee JD, et al. The clinical usefulness of 18-fluorodeoxyglucose positron emission tomography in the differential diagnosis, staging, and response evaluation after concurrent chemoradiotherapy for pancreatic cancer. J Clin Gastroenterol. 2006;40:923–9.
Belakhlef S, Church C, Hays A, Fraser R, Lakhanpal S. Quantitative assessment of the influence of location, internal temperature, idle time, and normalization on the sensitivity of a mobile PET/CT scanner. J Nucl Med Technol. 2008;36:147–50.
Watanuki S, Tashiro M, Miyake M, Ishikawa Y, Itoh M, Yanai K, et al. Long-term performance evaluation of positron emission tomography: analysis and proposal of a maintenance protocol for long-term utilization. Ann Nucl Med. 2010;24:461–8.
de Kemp R, Caldwell C, Farncombe T, McKee B, Wassenaar R, Wells R, et al. PET imaging standards and quality assurance for the multi-center trials of the Ontario Clinical Oncology Group. J Nucl Med. 2006;47:365P.
Mizuta T, Senda M, Okamura T, Kitamura K, Inaoka Y, Takahashi M, et al. NEC density and liver ROI S/N ratio for image quality control of whole-body FDG-PET scans: comparison with visual assessment. Mol Imaging Biol. 2009;11:480–6.
Fukukita H, Senda M, Terauchi T, Suzuki K, Daisaki H, Matsumoto K, et al. Japanese guideline for the oncology FDG-PET/CT data acquisition protocol: synopsis of version 1.0. Ann Nucl Med. 2010;24:325–34.
Strother SC, Casey ME, Hoffman EJ. Measuring PET scanner sensitivity: relating countrates to image signal-to-noise ratio using noise equivalent counts. IEEE Trans Nucl Sci. 1990;37:783–8.
Akamatsu G, Ishikawa K, Mitsumoto K, Taniguchi T, Ohya N, Baba S, et al. Improvement in PET/CT image quality with a combination of point-spread function and time-of-flight in relation to reconstruction parameters. J Nucl Med. 2012;53:1716–22.
Masuda Y, Kondo C, Matsuo Y, Uetani M, Kusakabe K. Comparison of imaging protocols for 18F-FDG PET/CT in overweight patients: optimizing scan duration versus administered dose. J Nucl Med. 2009;50:844–8.
Nagaki A, Onoguchi M, Matsumoto N. Patient weight-based acquisition protocols to optimize 18F-FDG PET/CT image quality. J Nucl Med Technol. 2011;39:72–6.
de Groot EH, Post N, Boellaard R, Wagenaar NR, Willemsen ATM, van Dalen JA. Optimized dose regimen for whole-body FDG-PET imaging. EJNMMI Res. 2013;3:63.
Geismar JH, Stolzmann P, Sah BR, Burger IA, Seifert B, Delso G, et al. Intra-individual comparison of PET/CT with different body weight-adapted FDG dosage regimens. Acta Radiol Open. 2015;4:1–9.
Yan J, Schaefferkoetter J, Conti M, Townsend D. A method to assess image quality for low-dose PET: analysis of SNR, CNR, bias and image noise. Cancer Imaging. 2016;16:26.
Irie S, Hayashida N, Shinkawa T, Kamasaki T, Matsunaga A, Miyamoto I, et al. Clinical usefulness of 18F-FDG PET/CT for the screening of metabolic liver disorders. Life Sci J. 2014;11:99–104.
Chang T, Chang G, Clark JW, Diab RH, Rohren E, Mawlawi OR. Reliability of predicting image signal-to-noise ratio using noise equivalent count rate in PET imaging. Med Phys. 2012;39:5891–900.
McDermott GM, Chowdhury FU, Scarsbrook AF. Evaluation of noise equivalent count parameters as indicators of adult whole-body FDG-PET image quality. Ann Nucl Med. 2013;27:855–61.
Viner M, Mercier G, Hao F, Malladi A, Subramaniam RM. Liver SULmean at FDG PET/CT: interreader agreement and impact of placement of volume of interest. Radiology. 2013;267:596–601.
Rudd JHF, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C, et al. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med. 2008;49:871–8.
Goh V, Shastry M, Engledow A, Kozarski R, Peck J, Endozo R, et al. Integrated 18F-FDG PET/CT and perfusion CT of primary colorectal cancer: effect of inter- and intraobserver agreement on metabolic-vascular parameters. AJR. 2012;199:1003–9.
Chang DW, Ersahin D, Staib LH, Della Latta D, Giorgetti A, d’Errico F. Using SUV as a guide to 18F-FDG dose reduction. J Nucl Med. 2014;55:1–5.
Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50:11S-20S.
Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of SUV measurements. AJR. 2010;195:310–20.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50:122S-150S.
Abikhzer G, Alabed YZ, Azoulay L, Assayag J, Rush C. Altered hepatic metabolic activity in patients with hepatic steatosis on FDG PET/CT. AJR 2011;196:176–80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were disclosed
Rights and permissions
About this article
Cite this article
Amakusa, S., Matsuoka, K., Kawano, M. et al. Influence of region-of-interest determination on measurement of signal-to-noise ratio in liver on PET images. Ann Nucl Med 32, 1–6 (2018). https://doi.org/10.1007/s12149-017-1215-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-017-1215-y