Abstract
Purpose
There is evidence that some cases of patients with dementia with Lewy bodies (DLB) can demonstrate Alzheimer disease (AD) like reduced glucose metabolism without amyloid deposition. The aim of this study was to clarify whether regional hypometabolism is related to amyloid deposits in the DLB brain and measure the degree of regional hypometabolism.
Methods
Ten consecutive subjects with DLB and 10 AD patients who underwent both Pittsburgh compound B (PiB)-PET and 18F-fluoro-2-deoxyglucose (FDG)-PET were included in this study. Regional standardized uptake value ratio (SUVR)s normalised to cerebellar cortices were calculated in the FDG- and PiB-PET images.
Results
All AD patients and five DLB patients showed amyloid deposits (PiB positive). In the DLB group the parietotemporal and occipital metabolism were significantly lower than those in the AD group but there was no difference between the posterior cingulate hypometabolism between DLB and AD groups. There were no differences in regional glucose metabolism between PiB positive and negative DLB patients.
Conclusions
In the DLB brain, it is suggested that decreased regional glucose metabolism is unrelated to amyloid deposits, although the hypometabolic area overlaps with the AD hypometabolic area and the degree of parietotemporal and occipital hypometabolism in DLB brain is much larger than that in AD brain.
Similar content being viewed by others
Introduction
Dementia with Lewy bodies (DLB) is the second most common neurodegenerative dementia following Alzheimer disease (AD). The clinical core features of DLB include fluctuating cognition, recurrent visual hallucinations, and Parkinsonism. DLB is characterised pathologically by the presence of Lewy bodies in cortical, subcortical, and brainstem structures, and belongs to the spectrum of Lewy body diseases including Parkinson disease (PD), where Lewy bodies remain in the brainstem. The criteria for clinical and pathological diagnoses of DLB were revised in 2005, and included an important role for functional and morphological imaging [1]. Suggestive features for DLB include low dopamine transporter uptake in the basal ganglia on SPECT and PET imaging scans, while supportive features include relative preservation of medial temporal lobe structures on CT/MRI scan and generalised low uptake on SPECT/PET with reduced occipital activity and abnormal MIBG myocardial scintigraphy [2].
The hypometabolic regions in DLB brains are similar to those in AD brains, although DLB brains exhibit a difference in the involved occipital lobe. For example, the relative metabolic reduction was reported to be more severe in the occipital cortices and less severe in the medial temporal lobes in DLB compared with AD, and occipital hypometabolism is a key feature of DLB that discriminates it from AD [3]. Donaghy et al. [4] also suggested that cortical amyloid deposition may be a factor in the development of cognitive impairment in ‘some’ cases of dementia in Lewy body disorders, and amyloid deposition may predict the future development of dementia in PD. Kantarci et al. [5] reported that occipital and posterior parietotemporal lobe hypometabolism in DLB is independent of the amyloid pathology. A correlation between amyloid deposition and symptom profile, severity, and progression remains controversial, as some patients with DLB can demonstrate a pattern of AD-like reduced glucose metabolism without amyloid deposition [5–7]. Thus, the aim of this study was to investigate the relationship between parietotemporal and posterior cingulate glucose metabolic reduction with amyloid deposition in the DLB brain and compare the degree of regional hypometabolism between DLB and AD brain.
Methods
Subjects
A total of 10 probable DLB patients (mean age 75.4 ± 7.5, male:female = 5:5, mean duration of the illness 2.05 years) were recruited from those who consulted our memory clinic and received 11C-PiB (Pittsburgh compound B)-PET from June 2011 to December 2013. Ten age- and sex-matched patients with AD (mean age 73.3 ± 5.3, male:female = 4:6, mean duration of the illness 1.75 years) were also examined from our Dementia database. All patients were examined by routine laboratory tests, structural neuroimaging (magnetic resonance imaging or computed tomography), and standard neuropsychological examinations, including the MMSE. The DLB patients fulfilled the criteria of the third report of the DLB Consortium [1], while the AD patients fulfilled the criteria of the NINCDS/ADRDA for probable AD [8]. This study was approved by the Ethics Committee of Kinki University Faculty of Medicine, and written informed consent was obtained for all participants. All subjects underwent 18F-fluoro-2-deoxyglucose (FDG)-PET within 30 days of 11C-PiB-PET scanning. In one DLB patient, amantadine was taken, in one DLB patient amantadine and levodopa were taken, in one DLB patient and two AD patients donepezil was taken, and in one AD patient donepezil and memantine were taken at the time of PET studies.
PiB-PET
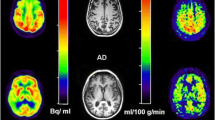
11C-PIB was synthesized at the PET Center of our institution as described previously [9]. PET scans were performed using a PET scanner (ECAT Accel, Siemens AG, Erlangen, Germany) in the 3D mode. A transmission scan was performed for correction of attenuation before administration of the tracer. 11C-PiB was injected into an antecubital vein, with a mean dose of 555 ± 185 MBq (11.1 MBq/kg body weight), and then the vein was flushed with saline. Images were acquired at 50–70 min after injection. The images were reconstructed with an iterative algorithm that provided spatial and axial resolution in the range of 6–8 mm at full-width and half-maximum. For visual inspection, standard uptake value images were obtained with a rainbow colour scale by normalizing the tissue radioactivity at 50–70 min after injection.
FDG-PET
For FDG-PET, the subjects were instructed to fast for at least 4 h prior to the scan and they were asked to lie quietly in a dimly lit room with their eyes open and minimal sensory stimulation. A 30 min dynamic emission scan (consisting of six 5 min frames) was acquired, starting 30 min after intravenous injection of 185 MBq of 18F-FDG. Data were corrected for attenuation by obtaining a transmission scan and images were reconstructed by the same method as for PiB images.
Image analysis
PiB-PET images of all subjects were visually assessed by two experienced nuclear medicine physicians blinded to the clinical information separately adjusting the window scale of the images. If two ratings were not consistent, consensus was achieved by discussion. PiB positivity was evaluated in cortical regions, including the bilateral frontal lobes, parietotemporal lobes, occipital lobes, precuneus/posterior cingulate gyri and striata. According to the PiB positivity in each region, each subject was classified as PiB positive, questionable, or PiB negative. For each region, PiB positive was defined as higher accumulation in the cerebral cortex than in the white matter, and PiB negative was defined as no cortical accumulation. When cortical accumulation was suspected but the accumulation in the cortex was not higher than that in the white matter, an equivocal rating was assigned. For the questionable cases, distribution of the PiB accumulation was classified as diffuse or focal among the four cortical regions.
In addition to visual reading, voxel-wise analyses were performed as follows. All of the PiB-PET images were co-registered to the individual FDG-PET images using statistical parametric mapping (SPM8: Wellcome Trust Centre for Neuroimaging, University College London, London, UK; http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). First, spatial normalisation of the FDG-PET images to the Montreal Neurologic Institute (MNI) space was performed with the SPM program. Next, spatial normalisation of the PiB-PET images to the MNI space was done using the individual parameter obtained from FDG-PET normalisation. Furthermore, all FDG-PET and PiB-PET images were smoothed with an isotropic 12 mm Gaussian kernel to increase the signal-to-noise ratio and to compensate for differences in gyral anatomy between individuals. The individual FDG images were normalised by the cerebellar FDG uptake. In the PiB analyses, the individual PiB uptake values were normalised by the cerebellar PiB uptake.
Voxel-by-voxel statistical analysis by SPM8 was conducted for comparisons of metabolic activity and PiB uptake between the DLB and AD subjects and between the PiB positive and negative DLB subjects. A significant threshold of p < 0.001, uncorrected for multiple comparisons, was applied. In addition, using the template volumes of interests (VOI) (frontal, parieto-temporal, posterior cingulated/precuneus, occipital, striatum, cerebellar cortices, and pons) set at the MNI space, regional FDG and PiB counts were measured for each region and then the regional SUVR of FDG and PiB were calculated relative to that of the cerebellar cortices as a reference. Concerning SUVR of FDG, cerebellar FDG uptake maybe affected, we additionally calculated SUVR of FDG relative to the pons. The differences of mean age and mean MMSE between the DLB group and AD group or between the PiB positive and PiB negative groups were examined with t tests and regional SUVR of FDG and PiB were examined with Tukey HSD test. The statistically significant level was set at p < 0.05.
Results
Table 1 displays the characteristics of the patients with DLB. The mean MMSE scores of the DLB and AD groups were 23.7 ± 3.8 and 21.5 ± 2.5, respectively. The mean MMSE score of AD group was similar to the DLB group (p = 0.07).
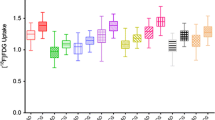
All patients with AD and five patients with DLB were PiB positive, one patient with DLB was PiB questionable, and four patients with DLB were PiB negative. In this study, a PiB questionable subject was included in the PiB negative group because in the majority of cortices there was no PiB uptake, except for the right temporal gyri. The mean MMSE score of PiB positive DLB group was 24.0 ± 2.3 and the mean MMSE score of PiB negative DLB group was 23.4 ± 5.1: there was no significant difference (p = 0.39). The right temporal and bilateral occipital metabolisms in the DLB group were significantly lower than those of the AD group (Fig. 1). In DLB patients, there were no significant differences in regional glucose metabolism between PiB positive and negative patients, and as a matter of course frontal, parietal and posterior cingulate, and precuneus PiB uptakes in the PiB positive group were significantly higher than those of the PiB negative group (Fig. 2).
For VOI analysis, the lateral parietotemporal and occipital metabolism in DLB group were significantly lower than those of AD group and striatal PiB uptake in DLB group was significantly lower than that of AD group: the group differences were the same, regardless of the reference region cerebellar cortices or pons (Table 2). In DLB patients, there were no significant regional glucose metabolism between PiB positive and negative patients and as a matter of course all regional PiB uptakes in the PiB positive group were significantly higher than those of PiB negative group (Table 3). PiB negative DLB group exhibited greater regional hypometabolism than AD group (Tables 2, 3). Parietotemporal, and occipital FDG uptakes in PiB negative DLB group were significantly lower than those in AD group but posterior cingulate and striatal FDG uptakes had no significant difference.
Discussion
Our study showed in DLB brain regional glucose metabolism is affected both in PiB positive and negative uptake subjects showing the similar reduction pattern of AD subjects and the degree of hypometabolism in parietotemporal and occipital cortices is larger than that of AD. This indicates that in DLB brain amyloid deposition has no correlation with reduced regional glucose metabolism as does in AD brain.
Kantarci et al. [5] reported that decreased occipital and posterior parietotemporal metabolism is a distinguishing feature of DLB from AD, and that this regional hypometabolic pattern is independent of the amyloid pathology. Our study verified this and also demonstrated severe parietotemporal hypometabolism in DLB brain compared with AD brain in spite of no amyloid deposition (Tables 2, 3). In the present study, we showed that there was no difference of posterior cingulate and precuneus hypometabolism between DLB group and AD group. Lim et al. [10] reported that relative preservation of the posterior cingulate gyrus (cingulate island sign) may enhance the specificity of FDG-PET in discriminating DLB from AD. There is a large overlap of cingulate metabolic reduction in DLB and AD brains, cingulate island sign might not be useful to discriminate DLB from AD.
There is some evidence that DLB patients have higher amyloid deposits than patients with PD and PD with dementia (PDD) [11, 12] and that amyloid deposit are associated with AD-like atrophy in DLB/PDD patients [11]. Gomperts et al. [12] suggested that as global cortical amyloid burden is high in DLB but low in PDD, then amyloid deposits may contribute selectively to the cognitive impairment of DLB and to the timing of dementia relative to the motor signs of Parkinsonism. Maetzler et al. [13] reported that patients with Lewy body disease accompanied by cortical amyloid deposits demonstrate characteristics usually observed in AD. In that study, the authors demonstrated cortical amyloid deposits in a relevant portion of patients with Lewy body disease, and then compared PiB binding with demographic, clinical, genetic, and biochemical parameters. However, they did not estimate the regional glucose metabolism in those subjects. Following these findings, it was considered the decreased glucose metabolism in the parietotemporal association cortices and posterior cingulate gyri and precuneus in the AD brain is associated with amyloid deposits. In the DLB brain, AD affected areas and occipital hypometabolism are commonly observed, and occipital hypometabolism is the characteristic feature that discriminates DLB from AD.
Using PiB-PET, Brooks et al. [14] reported that PDD may be differentiated from DLB by its relatively lower amyloid plaque load. Amyloid deposition may contribute to an increased severity of cognitive impairment in Lewy body disease at the stage from PD, then PDD to DLB. However, the percentage of PiB positive DLB patients in our study was lower compared to other studies [12, 15, 16]. This may also support that cognitive decline due to DLB pathology is independent from AD pathology, as there were no differences in regional hypometabolism between the PiB positive and negative DLB group in the present study.
Interestingly, a case of antemortem amyloid imaging and beta-amyloid pathology in a patient with DLB was previously reported [6]. The FDG-PET imaging of this subject demonstrated an advanced AD-like hypometabolic area, although PiB-PET imaging showed no uptake in the parietotemporal cortices and posterior cingulate cortices where glucose metabolism was severely decreased. This phenomenon was also observed in the PiB negative DLB patients in the present study. We suggest that neuropathological features other than amyloid, such as alpha-synucleinopathy, may contribute to the majority of the associated cognitive decline and regional hypometabolism in the DLB brain [17]. New imaging techniques capable of indicating alpha-synuclein are currently being developed.
In conclusion, despite the similar regional hypometabolism in the DLB brain compared to AD, the parietotemporal and posterior cingulate hypometabolism is unrelated to amyloid deposits and the degree of reduced metabolism is much larger in the parietotemporal cortices and there is no difference of posterior cingulate and precueus hypometabolism, although the cognitive decline is milder than that of AD.
References
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72.
Warr L, Walker Z. Identification of biomarkers in Lewy-body disorders. Q J Nucl Med Mol Imaging. 2012;56:39–54.
Ishii K, Imamura T, Sasaki M, Yamaji S, Sakamoto S, Kitagaki H, et al. Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer’s disease. Neurology. 1998;51:125–30.
Donaghy P, Thomas AJ, O’Brien JT. Amyloid PET Imaging in Lewy Body Disorders. Am J Geriatr Psychiatry. 2013 [Epub ahead of print].
Kantarci K, Lowe VJ, Boeve BF, Weigand SD, Senjem ML, Przybelski SA, et al. Multimodality imaging characteristics of dementia with Lewy bodies. Neurobiol Aging. 2012;33:2091–105.
Kantarci K, Yang C, Schneider JA, Senjem ML, Reyes DA, Lowe VJ, et al. Antemortem amyloid imaging and beta-amyloid pathology in a case with dementia with Lewy bodies. Neurobiol Aging. 2012;33:878–85.
Meyer PT, Frings L, Hellwig S. Update on SPECT and PET in parkinsonism—part 2: biomarker imaging of cognitive impairment in Lewy-body diseases. Curr Opin Neurol. 2014;27:398–404.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–44.
Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46:2740–54.
Lim SM, Katsifis A, Villemagne VL, Best R, Jones G, Saling M, et al. The 18F-FDG PET cingulate island sign and comparison to 123I-beta-CIT SPECT for diagnosis of dementia with Lewy bodies. J Nucl Med. 2009;50:1638–45.
Shimada H, Shinotoh H, Hirano S, Miyoshi M, Sato K, Tanaka N, et al. beta-Amyloid in Lewy body disease is related to Alzheimer’s disease-like atrophy. Mov Disord. 2013;28:169–75.
Gomperts SN, Rentz DM, Moran E, Becker JA, Locascio JJ, Klunk WE, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71:903–10.
Maetzler W, Liepelt I, Reimold M, Reischl G, Solbach C, Becker C, et al. Cortical PIB binding in Lewy body disease is associated with Alzheimer-like characteristics. Neurobiol Dis. 2009;34:107–12.
Brooks DJ. Imaging amyloid in Parkinson’s disease dementia and dementia with Lewy bodies with positron emission tomography. Mov Disord. 2009;24(Suppl 2):S742–7.
Edison P, Rowe CC, Rinne JO, Ng S, Ahmed I, Kemppainen N, et al. Amyloid load in Parkinson’s disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry. 2008;79:1331–8.
Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–25.
Colom-Cadena M, Gelpi E, Charif S, Belbin O, Blesa R, Marti MJ, et al. Confluence of alpha-synuclein, tau, and beta-amyloid pathologies in dementia with Lewy bodies. J Neuropathol Exp Neurol. 2013;72:1203–12.
Acknowledgments
The authors thank Mr. Yoshiyuki Nakayama for his support for brain FDG-PET and PiB-PET at Kinki University, and thank Dr. Kazushi Hanada, Prof. Osamu Shirakawa at Mental Healthcare Department, Kinki University Hospital, Dr. Masami Ueda, Dr. Kazuma Saigo, Prof. Susumu Kusunoki at Department of Neurology, Kinki University Hospital, and Dr. Makito Hirano at Department of Neurology, Kinki University Sakai Hospital for their support and providing clinical information. This study was supported in part by Japan Society for the Promotion of Science KAKENHI Grant Number 50534103 and the 21st Century Research and Development Incentive Wages at Kinki University.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ishii, K., Hosokawa, C., Hyodo, T. et al. Regional glucose metabolic reduction in dementia with Lewy bodies is independent of amyloid deposition. Ann Nucl Med 29, 78–83 (2015). https://doi.org/10.1007/s12149-014-0911-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-014-0911-0