Abstract
Background
Molecular diagnostics has greatly refined sinonasal tumor pathology over the past decade. While much of the attention has focused on carcinomas, it is becoming clear that there are emerging mesenchymal neoplasms which have previously defied classification.
Methods
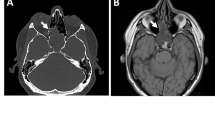
Here, we present a 33-year-old woman with a multiply recurrent sinonasal spindle cell tumor exhibiting distinctive features, and not easily classifiable into a specific category.
Results
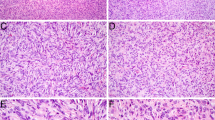
The hypercellular tumor was composed of plump spindled cells, with uniform vesicular chromatin arranged as vague fascicles around a prominent hemangiopericytoma-like vasculature. The mitotic rate was brisk at 10 per 10 high power fields. By immunohistochemistry, it was only positive for EMA (focal) and SATB2 (diffuse, weak). Fusion analysis uncovered EWSR1::BEND2, a fusion which is best known for being seen in astroblastoma, but which has not yet been reported in sarcomas.
Conclusion
This case underscores the utility of fusion analysis when confronted with a sinonasal spindle cell neoplasm which does not neatly fit into any specific category. It remains to be seen if EWSR1::BEND2 sinonasal sarcoma represents a distinct entity.



Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
Code Availability
Not applicable.
References
Thompson LDR, Bishop JA (2022) Update from the 5th Edition of the World Health Organization classification of head and neck tumors: nasal cavity, paranasal sinuses and skull base. Head Neck Pathol 16(1):1–18
Lewis JT, Oliveira AM, Nascimento AG, Schembri-Wismayer D, Moore EA, Olsen KD et al (2012) Low-grade sinonasal sarcoma with neural and myogenic features: a clinicopathologic analysis of 28 cases. Am J Surg Pathol 36(4):517–525
Le Loarer F, Laffont S, Lesluyes T, Tirode F, Antonescu C, Baglin AC et al (2019) Clinicopathologic and molecular features of a series of 41 biphenotypic sinonasal sarcomas expanding their molecular spectrum. Am J Surg Pathol 43(6):747–754
Stevens TM, Rooper LM, Bacchi CE, Fernandes IL, Antonescu CR, Gagan J et al (2022) Teratocarcinosarcoma-like and adamantinoma-like head and neck neoplasms harboring NAB2::STAT6: unusual variants of solitary fibrous tumor or novel tumor entities? Head Neck Pathol 16(3):746–754
Mechtersheimer G, Andrulis M, Delank KW, Volckmar AL, Zhang L, von Winterfeld M et al (2021) RREB1-MKL2 fusion in a spindle cell sinonasal sarcoma: biphenotypic sinonasal sarcoma or ectomesenchymal chondromyxoid tumor in an unusual site? Genes Chromosom Cancer 60(8):565–570
Rooper LM, Gagan J, Bishop JA (2022) A low grade nasopharyngeal sarcoma with FUS::NACC1 fusion and immunohistochemical evidence of epithelial differentiation: expanding the clinicopathologic spectrum of an emerging entity. Head Neck Pathol. https://doi.org/10.1007/s12105-022-01488-9
Garcia R, Patel N, Uddin N, Park JY (2020) Development and clinical validation of a multiplex gene fusion assay. Lab Med 51(5):512–518
Lucas CG, Gupta R, Wu J, Shah K, Ravindranathan A, Barreto J et al (2022) EWSR1-BEND2 fusion defines an epigenetically distinct subtype of astroblastoma. Acta Neuropathol 143(1):109–113
Todorovic E, Dickson BC, Weinreb I (2020) Salivary gland cancer in the era of routine next-generation sequencing. Head Neck Pathol 14(2):311–320
Scarpa A, Chang DK, Nones K, Corbo V, Patch AM, Bailey P et al (2017) Whole-genome landscape of pancreatic neuroendocrine tumours. Nature 543(7643):65–71
Yoshida A, Satomi K, Kobayashi E, Ryo E, Matsushita Y, Narita Y et al (2022) Soft-tissue sarcoma with MN1-BEND2 fusion: a case report and comparison with astroblastoma. Genes Chromosom Cancer 61(7):427–431
Moller E, Praz V, Rajendran S, Dong R, Cauderay A, Xing YH et al (2022) EWSR1-ATF1 dependent 3D connectivity regulates oncogenic and differentiation programs in clear cell sarcoma. Nat Commun 13(1):2267
Rossi S, Szuhai K, Ijszenga M, Tanke HJ, Zanatta L, Sciot R et al (2007) EWSR1-CREB1 and EWSR1-ATF1 fusion genes in angiomatoid fibrous histiocytoma. Clin Cancer Res 13(24):7322–7328
Antonescu CR, Katabi N, Zhang L, Sung YS, Seethala RR, Jordan RC et al (2011) EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosom Cancer 50(7):559–570
Ke H, Gill AJ, McKenzie C, Kench JG, Chan RCF, Pavlakis N et al (2021) Malignant peritoneal mesothelioma with EWSR1-ATF1 fusion: a case report. JTO Clin Res Rep 2(11):100236
Sturm D, Orr BA, Toprak UH, Hovestadt V, Jones DTW, Capper D et al (2016) New brain tumor entities emerge from molecular classification of CNS-PNETs. Cell 164(5):1060–1072
Yamasaki K, Nakano Y, Nobusawa S, Okuhiro Y, Fukushima H, Inoue T et al (2020) Spinal cord astroblastoma with an EWSR1-BEND2 fusion classified as a high-grade neuroepithelial tumour with MN1 alteration. Neuropathol Appl Neurobiol 46(2):190–193
Burford A, Mackay A, Popov S, Vinci M, Carvalho D, Clarke M et al (2018) The ten-year evolutionary trajectory of a highly recurrent paediatric high grade neuroepithelial tumour with MN1:BEND2 fusion. Sci Rep 8(1):1032
Zheng L, Liu J, Niu L, Kamran M, Yang AWH, Jolma A et al (2022) Distinct structural bases for sequence-specific DNA binding by mammalian BEN domain proteins. Genes Dev 36(3–4):225–240
Ma L, Xie D, Luo M, Lin X, Nie H, Chen J et al (2022) Identification and characterization of BEND2 as a key regulator of meiosis during mouse spermatogenesis. Sci Adv 8(21):eabn1606
Zhang J, Zhang Y, You Q, Huang C, Zhang T, Wang M et al (2022) Highly enriched BEND3 prevents the premature activation of bivalent genes during differentiation. Science 375(6584):1053–1058
Kurniawan F, Prasanth SG (2022) A BEN-domain protein and polycomb complex work coordinately to regulate transcription. Transcription 13(1–3):82–87
Funding
This study was funded by the Jane B. and Edwin P. Jenevein M.D Endowment for Pathology at UT Southwestern Medical Center. No external funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
VM and JAB designed the study, performed data collection and interpretation, and prepared the manuscript. JYP performed data collection and interpretation. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical Approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board (UT Southwestern IRB 112017-073).
Consent to Participate/Publication
The IRB-approved study did not require informed consent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Palsgrove, D.N., Manucha, V., Park, J.Y. et al. A Low-grade Sinonasal Sarcoma Harboring EWSR1::BEND2: Expanding the Differential Diagnosis of Sinonasal Spindle Cell Neoplasms. Head and Neck Pathol 17, 571–575 (2023). https://doi.org/10.1007/s12105-023-01527-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-023-01527-z