Abstract
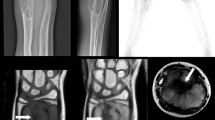
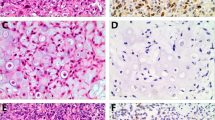
Benign fibro-osseous lesions (BFOLs) are a diverse group of lesions showing considerable degree of overlap with low grade osteosarcoma (LGOS). Further, de-differentiated osteosarcoma (DOS) is usually indistinguishable from conventional high-grade OS (COS) if LGOS foci are not identified. Thus, there is a need for adjunctive immunohistochemical markers to differentiate OS from benign FOLs as well as DOS from COS. This study evaluated the role of immunohistochemical expression of MDM2, CDK4, parafibromin, BCL-2 and Galectin-1 (Gal-1) in accurate characterization of benign FOLs and in differentiating them from OS. From our archives, we retrieved 101 tissue samples which were diagnosed as osteosarcoma (OS) /ossifying fibroma (OF) / fibrous dysplasia (FD) or fibrous hyperplasia (FH) and examined their immunohistochemical staining pattern with the aforementioned antibodies. MDM2 showed 100% specificity for diagnosing OS. CDK4 and Gal-1 showed linear increase in immunoexpression from benign BFOLs to OS. BCL-2 showed equivocal immunopositivity in OF and OS, but the positivity was higher than that observed in FD. The highest immunoexpression for parafibromin was seen in FD followed by OF and OS cases. Thus, MDM2 is most specific, and Gal-1 is most sensitive of all the markers studied in differentiating OS from benign mimics. Combination of these two markers can be used as an adjunct to conventional imaging and microscopy in accurate characterization of these lesions. Further MDM2 overexpression can differentiate DOS and COS.





Similar content being viewed by others
Data availability
Available.
Code availability
All the cases enrolled in the study were coded and the data is available.
References
Junior AT, de Abreu AF, Pinto CAL, Carvalho AL, Kowalski LP, Lopes MA. Clinicopathological and immunohistochemical analysis of twenty-five head and neck osteosarcomas. Oral Oncol. 2003;39(5):521–30.
Dujardin F, Binh MBN, Bouvier C, Gomez-Brouchet A, Larousserie F, de Muret A, et al. MDM2 and CDK4 immunohistochemistry is a valuable tool in the differential diagnosis of low-grade osteosarcomas and other primary fibro-osseous lesions of the bone. Mod Pathol. 2011;24(5):624–37.
Yoshida A, Ushiku T, Motoi T, Beppu Y, Fukayama M, Tsuda H, et al. MDM2 and CDK4 immunohistochemical coexpression in high-grade osteosarcoma: correlation with a dedifferentiated subtype. Am J Surg Pathol. 2012;36(3):423–31.
Bertoni F, Bacchini P, Staals EL, Davidovitz P. Dedifferentiated parosteal osteosarcoma: the experience of the Rizzoli Institute. Cancer. 2005;103(11):2373–82.
Sheth DS, Yasko AW, Raymond AK, Ayala AG, Carrasco CH, Benjamin RS, et al. Conventional and dedifferentiated parosteal osteosarcoma diagnosis, treatment, and outcome. Cancer. 1996;78(10):2136–45.
Tabareau-Delalande F, Collin C, Gomez-Brouchet A, Bouvier C, Decouvelaere A-V, de Muret A, et al. Chromosome 12 long arm rearrangement covering MDM2 and RASAL1 is associated with aggressive craniofacial juvenile ossifying fibroma and extracranial psammomatoid fibro-osseous lesions. Mod Pathol. 2015;28(1):48–56.
Lopes MA, Nikitakis NG, Ord RA, Sauk J. Amplification and protein expression of chromosome 12q13-15 genes in osteosarcomas of the jaws. Oral Oncol. 2001;37(7):566–71.
Yoshida A, Ushiku T, Motoi T, Shibata T, Beppu Y, Fukayama M, et al. Immunohistochemical analysis of MDM2 and CDK4 distinguishes low-grade osteosarcoma from benign mimics. Mod Pathol. 2010;23(9):1279–88.
Chen CY, Zhang HZ, Jiang ZM, Zhou J, Chen J, Liu L. Value of MDM2, CDK4 and SATB2 immunohistochemistry in histologic diagnosis of low-grade osteosarcoma. Zhonghua Bing Li Xue Za Zhi. 2016;45(6):387–92.
Gamberi G, Ragazzini P, Benassi MS, Ferrari C, Sollazzo MR, Molendini L, et al. Analysis of 12q13-15 genes in parosteal osteosarcoma. Clin Orthop. 2000;377:195–204.
Park H-R, Jung WW, Bertoni F, Bacchini P, Park JH, Kim Y-W, et al. Molecular analysis of p53, MDM2 and H-ras genes in low-grade central osteosarcoma. Pathol Res Pract. 2004;200(6):439–45.
Limbach AL, Lingen MW, McElherne J, Mashek H, Fitzpatrick C, Hyjek E, et al. The utility of MDM2 and CDK4 immunohistochemistry and MDM2 FISH in craniofacial osteosarcoma. Head Neck Pathol. 2020;14(4):889–98.
de Mesquita Netto AC, Gomez RS, Diniz MG, Fonseca-Silva T, Campos K, De Marco L, et al. Assessing the contribution of HRPT2 to the pathogenesis of jaw fibrous dysplasia, ossifying fibroma, and osteosarcoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(3):359–67.
Carpten JD, Robbins CM, Villablanca A, Forsberg L, Presciuttini S, Bailey-Wilson J, et al. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet. 2002;32(4):676–80.
Pimenta FJ, Gontijo Silveira LF, Tavares GC, Silva AC, Perdigão PF, Castro WH, et al. HRPT2 gene alterations in ossifying fibroma of the jaws. Oral Oncol. 2006;42(7):735–9.
Miettinen M, Sarlomo-Rikala M, Kovatich AJ. Cell-type- and tumour-type-related patterns of bcl-2 reactivity in mesenchymal cells and soft tissue tumours. Virchows Arch Int J Pathol. 1998;433(3):255–60.
Nedelcu T, Kubista B, Koller A, Sulzbacher I, Mosberger I, Arrich F, et al. Livin and Bcl-2 expression in high-grade osteosarcoma. J Cancer Res Clin Oncol. 2008;134(2):237–44.
Trieb K, Sulzbacher I, Kubista B. Bcl-2 correlates with localization but not outcome in human osteosarcoma. Oncol Lett. 2013;6(2):559–61.
Piro F, Leonardi L. Expression of Bcl-2 in canine osteosarcoma. Open Vet J. 2015;5(1):27–9.
Goud NS, Soukya PSL, Ghouse M, Komal D, Alvala R, Alvala M. Human galectin-1 and its inhibitors: privileged target for cancer and HIV. Mini Rev Med Chem. 2019;19(16):1369–78.
Chou F-C, Chen H-Y, Kuo C-C, Sytwu H-K. Role of galectins in tumors and in clinical immunotherapy. Int J Mol Sci. 2018;19(2):430.
Brito LNS, de Lemos Almeida MMR, de Souza LB, Alves PM, Nonaka CFW, Godoy GP. Immunohistochemical analysis of galectins-1, -3, and -7 in periapical granulomas, radicular cysts, and residual radicular cysts. J Endod. 2018;44(5):728–33.
Wold LE, Unni KK, Beabout JW, Sim FH, Dahlin DC. Dedifferentiated parosteal osteosarcoma. J Bone Joint Surg Am. 1984;66(1):53–9.
Kenan S, Ginat DT, Steiner GC. Dedifferentiated high-grade osteosarcoma originating from low-grade central osteosarcoma of the fibula. Skeletal Radiol. 2007;36(4):347–51.
Shuhaibar H, Friedman L. Dedifferentiated parosteal osteosarcoma with high-grade osteoclast-rich osteogenic sarcoma at presentation. Skeletal Radiol. 1998;27(10):574–7.
Lonardo F, Ueda T, Huvos AG, Healey J, Ladanyi M. p53 and MDM2 alterations in osteosarcomas: correlation with clinicopathologic features and proliferative rate. Cancer. 1997;79(8):1541–7.
Mejia-Guerrero S, Quejada M, Gokgoz N, Gill M, Parkes RK, Wunder JS, et al. Characterization of the 12q15 MDM2 and 12q13-14 CDK4 amplicons and clinical correlations in osteosarcoma. Genes Chromosomes Cancer. 2010;49(6):518–25.
Wei G, Lonardo F, Ueda T, Kim T, Huvos AG, Healey JH, et al. CDK4 gene amplification in osteosarcoma: reciprocal relationship with INK4A gene alterations and mapping of 12q13 amplicons. Int J Cancer. 1999;80(2):199–204.
Binh MBN, Sastre-Garau X, Guillou L, de Pinieux G, Terrier P, Lagacé R, et al. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: a comparative analysis of 559 soft tissue neoplasms with genetic data. Am J Surg Pathol. 2005;29(10):1340–7.
Guérin M, Thariat J, Ouali M, Bouvier C, Decouvelaere A-V, Cassagnau E, et al. A new subtype of high-grade mandibular osteosarcoma with RASAL1/MDM2 amplification. Hum Pathol. 2016;50:70–8.
Park H-R, Jung WW, Bertoni F, Bacchini P, Park JH, Kim Y-W, et al. Molecular analysis of p53, MDM2 and H-ras genes in low-grade central osteosarcoma. Pathol Res Pract. 2004;200(6):439–45.
Toki S, Kobayashi E, Yoshida A, Ogura K, Wakai S, Yoshimoto S, et al. A clinical comparison between dedifferentiated low-grade osteosarcoma and conventional osteosarcoma. Bone Jt J. 2019;101(6):745–52.
Ferrari S, Bertoni F, Zanella L, Setola E, Bacchini P, Alberghini M, et al. Evaluation of P-glycoprotein, HER-2/ErbB-2, p53, and Bcl-2 in primary tumor and metachronous lung metastases in patients with high-grade osteosarcoma. Cancer. 2004;100(9):1936–42.
Satelli A, Rao PS, Gupta PK, Lockman PR, Srivenugopal KS, Rao US. Varied expression and localization of multiple galectins in different cancer cell lines. Oncol Rep. 2008;19(3):587–94.
Funding
This research was funded by intramural grant of All India Institute of Medical Sciences, Delhi, India (A-626).
Author information
Authors and Affiliations
Contributions
DM: conceived the idea. DM and HK: designed the study. HK, DM, ARM, SM, AS, RY: collected patient’s data. HK, DM, AK, ARM, SK, AS: performed the pathological analysis. SM and AS: analyzed the data. DM: cross checked the study results. HK, DM: wrote the manuscript; ARM, SM, AS: edited the manuscript. RY, AK, SK: provided revisions to the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors have no conflict of interests to declare.
Ethical approval
Obtained.
Consent to participate
Obtained.
Consent for publication
Obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaur, H., Kala, S., Sood, A. et al. Role of MDM2, CDK4, BCL2, Parafibromin and Galectin 1 in Differentiating Osteosarcoma from its Benign Fibro-osseous Lesions. Head and Neck Pathol 16, 728–737 (2022). https://doi.org/10.1007/s12105-022-01434-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-022-01434-9