Abstract
The neuronal L-type voltage-gated Ca2+ channel (CaV1.2) interacts with Ca2+ binding protein 1 (CaBP1), that promotes Ca2+-induced channel activity. The binding of CaBP1 to the IQ-motif in CaV1.2 (residues 1644–1665) blocks the binding of calmodulin and prevents Ca2+-dependent inactivation of CaV1.2. This Ca2+-induced binding of CaBP1 to CaV1.2 is important for modulating neuronal synaptic plasticity, which may serve a role in learning and memory. Here we report NMR assignments of the C-terminal domain of CaBP1 (residues 99–167, called CaBP1C) that contains two Ca2+ bound at the third and fourth EF-hands (EF3 and EF4) and is bound to the CaV1.2 IQ-motif from CaV1.2 (BMRB accession no. 51518).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Biological context
CaV1.2 controls the excitability of the postsynaptic membrane in hippocampal neurons, which is important for learning and memory (Hell et al. 1993; Moosmang et al. 2005; Vogl et al. 2015). The cytosolic C-terminal region of CaV1.2 (residues 1644–1665, called IQ-motif) is important for promoting Ca2+-dependent inactivation (CDI) of CaV1.2 (Erickson et al. 2001; Zuhlke et al. 1999). Ca2+-free CaM has been suggested to bind to the IQ-motif to increase the channel open probability under basal conditions (Adams et al. 2014; Erickson et al. 2001). CaV1.2 channel opening promotes a rise in intracellular Ca2+ sensed by CaM that causes a Ca2+-induced conformational change in the CaM/CaV1.2 complex leading to CDI (Ben Johny et al. 2013; Peterson et al. 1999; Zuhlke et al. 1999). CaBP1 competes with CaM for binding to the IQ-motif (Findeisen et al. 2013; Hardie and Lee 2016), which prevents channel pre-association of CaM and abolishes CDI (Hardie and Lee 2016; Oz et al. 2011). Thus, CaBP1 serves as a competitive inhibitor of CDI and promotes constitutive channel activation at high Ca2+ levels (Hardie and Lee 2016; Oz et al. 2011), in contrast to CaM that causes Ca2+-induced channel inactivation (Peterson et al. 1999; Zuhlke et al. 1999). In essence, CaBP1 and CaM oppositely regulate CaV1.2 channel activity by serving as an accelerator and brake, respectively (Ames 2021). We report NMR resonance assignments for the C-terminal domain of CaBP1 (CaBP1C) with two Ca2+ bound that is bound to the IQ-motif of CaV1.2 (called CaBP1C-IQ) as a first step toward elucidating the intermolecular contacts between CaBP1 and CaV1.2.
Methods and experiments
Preparation of CaBP1C bound to the Ca V 1.2 IQ-motif
A cDNA of Homo sapiens CaBP1C was subcloned into pET-11b vector (Novagen) that produced recombinant CaBP1C without any extra residues. Recombinant CaBP1C uniformly labeled with 13C and 15N was expressed in bacterial cells grown on M9 minimal media supplemented with 15N-labeled NH4Cl (1 g per liter of cell culture) and 13C-labeled glucose (3 g per liter). The isotopically labeled CaBP1C was purified as described previously (Li et al. 2009). A peptide representing the CaV1.2 IQ-motif (residues 1642–1665) was purchased from GenScript, dissolved in DMSO-d6 and quantified using UV–Vis absorption. A 2.0-fold excess of IQ peptide was added to Ca2+-bound CaBP1C and the complex was concentrated to 500 μM in the presence of 2 mM CaCl2 using a 3 K Amicon concentrator.
NMR spectroscopy
Samples of CaBP1C-IQ for NMR were prepared by exchanging the complex into a buffer containing 20 mM Tris-d11 (pH 7.5) with 2 mM CaCl2, and 92% H2O/8% D2O. All NMR experiments were performed at 308 K on a Bruker Avance 600 MHz spectrometer equipped with a four-channel interface and triple resonance cryogenic (TCI) probe. The 15N-1H HSQC spectrum (Fig. 1) contained 256 × 2048 complex points for 15N(F1) and 1H(F2), respectively. Backbone resonances were assigned by analyzing HNCA, HNCACB, CBCA(CO)NH, HNCO (Ikura et al. 1990). Side chain resonances were assigned by analyzing HCCCONH-TOCSY, HCCH-TOCSY as described previously (Ikura et al. 1991). The NMR data were processed using NMRFx Analyst (Norris et al. 2016) and analyzed using Sparky NMRFAM (Lee et al. 2015).
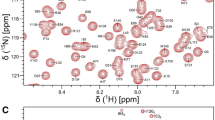
A Two-dimensional 15N-1H HSQC NMR spectrum of 15N-labeled full-length CaBP1 (blue) and CaBP1C (red) both bound to unlabeled CaV1.2 IQ peptide at pH 7.5 recorded at 600-MHz 1H frequency. B Constant-time 13C-1H HSQC spectrum of 13C-labeled CaBP1C bound to unlabeled IQ peptide. C Expanded view of the spectrally crowded region from panel A. Representative assignments are indicated by the labeled peaks; complete assignments are available as BMRB accession no. 51518
Extent of assignments and data deposition
Ideally, we would want to perform NMR structural analysis of the full-length CaBP1 bound to the CaV1.2 IQ. Unfortunately, the full-length CaBP1 forms a dimer and other oligomeric species at the protein concentrations required for 3D NMR, and the relatively large size of the protein dimer reduces the sensitivity of the triple resonance 3D NMR experiments. Instead, we chose to perform NMR structural analysis of the C-terminal domain of CaBP1 (residues 99–167, called CaBP1C) that is monomeric in solution (Li et al. 2009) and exhibits functional binding to the IQ peptide (Fig. 1A). A two-dimensional 15N-1H HSQC NMR spectrum of full-length CaBP1 bound to the IQ peptide (at 0.05 mM protein concentration where CaBP1-IQ is monomeric) demonstrates that the chemical shifts of the CaBP1 C-terminal domain are similar to those of CaBP1C bound to the IQ (Fig. 1A, C). The chemical shifts of the C-terminal domain residues of CaBP1 bound to the IQ are quite different from those of CaBP1 in the absence of the IQ, demonstrating that the IQ peptide binds to the CaBP1 C-terminal domain (Wingard et al. 2005). Also, the chemical shifts of the N-terminal domain residues of CaBP1 bound to the IQ (Fig. 1A) are the same (within experimental error) as those of CaBP1 in the absence of the IQ (Wingard et al. 2005). These results demonstrate that the IQ peptide binds solely to the C-terminal domain of CaBP1 (without contacting the N-terminal domain), and IQ binding to CaBP1C is structurally similar to that of full-length CaBP1.
The two-dimensional 15N-1H HSQC NMR spectrum of 15N-labeled CaBP1C bound to unlabeled CaV1.2 IQ peptide (called CaBP1C-IQ) illustrates representative NMR assignments (labeled red peaks in Fig. 1A). NMR assignments were derived from triple resonance NMR experiments performed on 13C/15N-labeled CaBP1C bound to unlabeled IQ peptide. The high level of chemical shift dispersion indicates that CaBP1C-IQ complex is stably folded. The large downfield chemical shifts of the amide resonances assigned to G117 and G154 confirm that Ca2+ is bound to EF3 and EF4 in CaBP1C (Fig. 1A). Other noteworthy downfield shifts were assigned to I119, S120 and V156 that are located in the EF-hand β-strands and are predicted to form antiparallel β-sheets with strong backbone amide hydrogen bonds. The upfield-shifted chemical shifts of methyl resonances assigned to residues I119, I141 and V162 (Fig. 1B) suggest that these residues may be located in the hydrophobic core near aromatic residues. More than 87% of the main chain 13C resonances (13Cα, 13Cβ, and 13CO), 85% of backbone amide resonances (1HN, 15N), and 74% of methyl side chain resonances were assigned. Side chain aromatic resonances from F108, F111, F158, and F161 appear exchange broadened in the NMR spectra and could not be assigned. The unassigned amide resonances from non-proline residues (110, 122, 125, 132, 134, 138, 139, 140, 141, 142, 143, 159, 164) had weak or missing NMR intensities that prevented their assignment. In particular, a stretch of residues between EF3 and EF4 (residues 138–143) could not be assigned due to weak NMR intensities, perhaps because this linker region is conformationally disordered or otherwise unstructured. A complete list of the chemical shift assignments (1H, 15N, 13C) of CaBP1C-IQ have been deposited in the BioMagResBank under accession number 51518.
The secondary structure of CaBP1C bound to the IQ peptide was calculated on the basis of chemical shift index (Wishart et al. 1992) and ANN-secondary structure prediction using TALOS (Shen et al. 2009) (Fig. 2). As expected, CaBP1C-IQ contains two EF-hands with four α-helices: α1 (residues 101–110), α2 (residues 121–132), α3 (residues 140–148) and α4 (residues 158–165) shown as green cylinders in Fig. 2. Conserved β-strands were observed in EF3 (residues 118–120, β1) and EF4 (residues 155–157, β2) shown as blue arrows in Fig. 2. The overall secondary structure of CaBP1C-IQ is similar to that of the crystal structure of CaBP1 (Findeisen and Minor 2010). A plot of the amide chemical shift perturbation caused by the binding of the IQ peptide reveals that CaBP1C residues F111, E126, V136, R146, V148, E160 and M165 exhibit the largest CSP values (Fig. 3A). Hydrophobic residues F111, V136, V148 and M165 are located in an exposed cleft in the crystal structure that are likely making intermolecular hydrophobic contacts with the IQ peptide (Fig. 3B). The NMR assignments of CaBP1C bound to the CaV1.2 IQ-motif are a first step toward determining the three-dimensional structure of CaBP1 bound to CaV1.2.
A Amide chemical shift perturbation for CaBP1C caused by the binding of IQ peptide. Chemical shift perturbation (CSP) was calculated as CSP = {(HNA–HNB)2 + (15NA–15NB)2}1/2. HNA and HNB are HN chemical shift of CaBP1C in the presence versus absence of IQ peptide, and 15NA and 15NB are 15N chemical shift of CaBP1C in the presence versus absence of IQ peptide. Chemical shifts of CaBP1C (in the absence of IQ) were obtained from BMRB 15623. B Crystal structure of the C-terminal domain of CaBP1 (Findeisen and Minor 2010). Exposed hydrophobic residues with the largest chemical shift perturbation are highlighted in red
Data availability
The assignments have been deposited to the BMRB under the accession code: 51518.
References
Adams PJ, Ben-Johny M, Dick IE, Inoue T, Yue DT (2014) Apocalmodulin itself promotes ion channel opening and Ca(2+) regulation. Cell 159:608–622
Ames JB (2021) L-type Ca(2+) channel regulation by calmodulin and CaBP1. Biomolecules 11:1811
Ben Johny M, Yang PS, Bazzazi H, Yue DT (2013) Dynamic switching of calmodulin interactions underlies Ca2+ regulation of CaV.13 channels. Nat Commun 4:1717
Erickson M, Alseikhan B, Peterson B, Yue D (2001) Preassociation of calmodulin with voltage-gated Ca(2+) channels revealed by FRET in single living cells. Neuron 31:973–985
Findeisen F, Minor DL (2010) Structural basis for the differential effects of CaBP1 and calmodulin on CaV1.2 calcium-dependent inactivation. Structure 18:1617–1631
Findeisen F, Rumpf CH, Minor DL Jr (2013) Apo states of calmodulin and CaBP1 control CaV1 voltage-gated calcium channel function through direct competition for the IQ domain. J Mol Biol 425:3217–3234
Hardie J, Lee A (2016) Decalmodulation of Cav1 channels by CaBPs. Channels 10:33–37
Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA (1993) Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol 123:949–962
Ikura M, Kay LE, Bax A (1990) A novel approach for sequential assignment of 1H, 13C, and 15N spectra of proteins: heteronuclear triple-resonance three-dimensional NMR spectroscopy. Appl Calmodulin Biochem 29:4659–4667
Ikura M, Spera S, Barbato G, Kay LE, Krinks M, Bax A (1991) Secondary structure and side-chain 1H and 13C resonance assignments of calmodulin in solution by heteronuclear multidimensional NMR spectroscopy. Biochemistry 30:9216–9228
Lee W, Tonelli M, Markley JL (2015) NMRFAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinformatics 31:1325–1327
Li C, Chan J, Haeseleer F, Mikoshiba K, Palczewski K, Ikura M, Ames JB (2009) Structural insights into Ca2+-dependent regulation of inositol 1,4,5-trisphosphate receptors by CaBP1. J Biol Chem 284:2472–2481
Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Muller J, Stiess M, Marais E, Schulla V, Lacinova L, Goebbels S, Nave K, Storm D, Hofmann F, Kleppisch T (2005) Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci 25:9883–9892
Norris M, Fetler B, Marchant J, Johnson BA (2016) NMRFx Processor: a cross-platform NMR data processing program. J Biomol NMR 65:205–216
Oz S, Tsemakhovich V, Christel CJ, Lee A, Dascal N (2011) CaBP1 regulates voltage-dependent inactivation and activation of Ca(V)1.2 (L-type) calcium channels. J Biol Chem 286:13945–13953
Peterson B, DeMaria C, Adelman J, Yue D (1999) Calmodulin is the Ca2+ sensor for Ca2+ -dependent inactivation of L-type calcium channels. Nature 22:549–558
Shen Y, Delaglio F, Cornilescu G, Bax A (2009) TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR 44:213–223
Vogl AM, Brockmann MM, Giusti SA, Maccarrone G, Vercelli CA, Bauder CA, Richter JS, Roselli F, Hafner AS, Dedic N, Wotjak CT, Vogt-Weisenhorn DM, Choquet D, Turck CW, Stein V, Deussing JM, Refojo D (2015) Neddylation inhibition impairs spine development, destabilizes synapses and deteriorates cognition. Nat Neurosci 18:239–251
Wingard JN, Chan J, Bosanac I, Haeseleer F, Palczewski K, Ikura M, Ames JB (2005) Structural analysis of Mg2+ and Ca2+ binding to CaBP1, a neuron-specific regulator of calcium channels. J Biol Chem 280:37461–37470
Wishart DS, Sykes BD, Richards FM (1992) The chemical shift index: a fast and simple method for the assignment of protein secondary structure through NMR spectroscopy. Biochemistry 31:1647–1651
Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H (1999) Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature 399:159–162
Acknowledgements
We thank Derrick Kaseman and Ping Yu for technical support and help with NMR experiments performed at the UC Davis NMR Facility.
Funding
Work supported by NIH grants to J.B.A (R01 GM130925) and to the UC Davis NMR Facility (RR11973).
Author information
Authors and Affiliations
Contributions
IS performed all experiments, analyzed data and helped write the manuscript. JBA directed the overall project and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no competing conflict of interest.
Ethical approval
The experiments comply with the current laws of the United States.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salveson, I., Ames, J.B. Chemical shift assignments of the C-terminal domain of CaBP1 bound to the IQ-motif of voltage-gated Ca2+ channel (CaV1.2). Biomol NMR Assign 16, 385–390 (2022). https://doi.org/10.1007/s12104-022-10108-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-022-10108-0