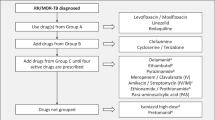
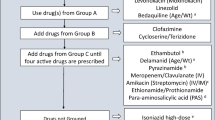
Abstract
In September 2022, the World Health Organization (WHO) published a new guideline for the management of tuberculosis (TB) in children and adolescents. It included eight new recommendations. Xpert MTB/RIF Ultra (Xpert Ultra) has been designated as the preferred initial diagnostic test for pulmonary TB and detection of rifampicin resistance. But its place vis-à-vis the previously recommended GeneXpert has not been clarified. Further, the limited diagnostic accuracy of Xpert Ultra in some biological specimens like nasopharyngeal aspirates, and the inability to report the presence or absence of rifampicin resistance in ‘trace’ reports has not been addressed. The guideline also recommends a shortened 4-mo treatment regimen for non-severe drug-susceptible TB. This is based on a single trial having several methodological issues that limit its applicability and generalizability. Interestingly, the criteria for designating ‘non-severe’ TB in the trial is based on smear negativity, whereas the new WHO recommendation is to omit smear microscopy altogether. The guideline also recommends an alternative 6-mo intensive regimen for drug-susceptible TB meningitis, which needs more supportive evidence. The lower age limits for the use of bedaquiline and delamanid have been decreased to less than 6 and 3 y respectively. While this makes it feasible to treat drug resistant TB in children with oral medications, the resource implications need careful consideration. These concerns advocate caution before the WHO guideline recommendations can be universally implemented.
Similar content being viewed by others
References
World Health Organization. WHO Consolidated Guidelines on Tuberculosis: Module 5: Management of Tuberculosis in Children and Adolescents [Internet]. Available at: https://www.who.int/publications/i/item/9789240046764. Accessed on 10th Oct 2022.
World Health Organization. Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children, 2nd ed [Internet]. Available at: https://www.who.int/publications/i/item/9789241548748. Accessed on 10th Oct 2022.
World Health Organization. WHO Consolidated Guidelines on Tuberculosis. Module 3: Diagnosis - Rapid Diagnostics for Tuberculosis Detection, 2021 Update. Available at: https://www.who.int/publications/i/item/9789240029415. Accessed on 10th Oct 2022.
Singh V. Pediatric TB Management under RNTCP: what and why? Indian J Pediatr. 2019;86:707–13.
Kay AW, González Fernández L, Takwoingi Y, et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children. Cochrane Database Syst Rev. 2020;8:CD013359.
Chakravorty S, Simmons AM, Rowneki M, et al. The New Xpert MTB/RIF Ultra: improving detection of mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. mBio. 2017;8:e00812–17.
Jaganath D, Wambi P, Reza TF, et al. A prospective evaluation of Xpert MTB/RIF Ultra for Childhood Pulmonary Tuberculosis in Uganda. J Pediatr Infect Dis Soc. 2021;10:586–92.
Ssengooba W, Iragena JD, Nakiyingi L, et al. Accuracy of Xpert ultra in diagnosis of pulmonary tuberculosis among children in uganda: a substudy from the SHINE Trial. J Clin Microbiol. 2020;58:e00410–20.
Kabir S, Rahman SMM, Ahmed S, et al. Xpert Ultra assay on stool to diagnose pulmonary tuberculosis in children. Clin Infect Dis. 2021;73:226–34.
Liu XH, Xia L, Song B, et al. Stool-based Xpert MTB/RIF ultra assay as a tool for detecting pulmonary tuberculosis in children with abnormal chest imaging: a prospective cohort study. J Infect. 2021;82:84–9.
The Union’s desk guide for diagnosis and management of TB in children. Third edition, 2016. Paris: International Union Against Tuberculosis and Lung Disease; 2016. Available at: https://theunion.org/sites/default/files/2020-08/2016_Desk-guide_Africa_Web.pdf. Accessed on 23rd Jan 2023.
Graham SM, Cuevas LE, Jean-Philippe P, et al. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clin Infect Dis. 2015;61:179–87.
World Health Organization. WHO Consolidated Guidelines on Tuberculosis: Module 5: Management of Tuberculosis in Children and Adolescents: Web Annex 3. GRADE Evidence to Decision Tables. Available at: https://apps.who.int/iris/bitstream/handle/10665/352509/9789240046801-eng.pdf. Accessed on 23rd Jan 2023.
World Health Organization. WHO Operational Handbook on Tuberculosis: Module 5: Management of Tuberculosis in Children and Adolescents [Internet]. Available at: https://www.who.int/publications/i/item/9789240046832. Accessed on 10th Dec 2022.
Turkova A, Wills GH, Wobudeya E, et al. Shorter treatment for nonsevere tuberculosis in African and indian children. N Engl J Med. 2022;386:911–22.
Agarwal G, Awasthi S, Kabra SK, et al. Three day versus five day treatment with amoxicillin for non-severe pneumonia in young children: a multicentre randomised controlled trial. BMJ. 2004;328:791.
Ginsburg AS, Mvalo T, Nkwopara E, et al. Amoxicillin for 3 or 5 days for chest-indrawing pneumonia in malawian children. N Engl J Med. 2020;383:13–23.
Pakistan Multicentre Amoxycillin Short Course Therapy (MASCOT) pneumonia study group. Clinical efficacy of 3 days versus 5 days of oral amoxicillin for treatment of childhood pneumonia: a multicentre double-blind trial. Lancet. 2002;360:835–41.
Department of Health, Republic of South Africa. Guidelines for the management of tuberculosis in children. Pretoria, South Africa: Department of Health; 2013. Available at: https://www.knowledgehub.org.za/system/files/elibdownloads/2020-04/National-Childhood-TB-Guidelines-2013-ZA.pdf. Accessed on 23rd Dec 2022.
Donald PR. The chemotherapy of tuberculous meningitis in children and adults. Tuberculosis (Edinb). 2010;90:375–92.
Bang ND, Caws M, Truc TT, et al. Clinical presentations, diagnosis, mortality and prognostic markers of tuberculous meningitis in vietnamese children: a prospective descriptive study. BMC Infect Dis. 2016;16:573.
van Well GT, Paes BF, Terwee CB, et al. Twenty years of pediatric tuberculous meningitis: a retrospective cohort study in the western cape of South Africa. Pediatrics. 2009;123:e1–8.
van Toorn R, Schaaf HS, Laubscher JA, van Elsland SL, Donald PR, Schoeman JF. Short intensified treatment in children with drug-susceptible tuberculous meningitis. Pediatr Infect Dis J. 2014;33:248–52.
Thee S, Basu Roy R, Blázquez-Gamero D, et al. Treatment and outcome in children with tuberculous meningitis: a multicenter pediatric tuberculosis network european trials group study. Clin Infect Dis. 2022;75:372–81.
Gupta R, Kushwaha S, Thakur R, et al. Predictors of adverse outcome in patients of tuberculous meningitis in a multi-centric study from India. Indian J Tuberc. 2017;64:296–301.
Dhawan SR, Gupta A, Singhi P, Sankhyan N, Malhi P, Khandelwal N. Predictors of neurological outcome of tuberculous meningitis in childhood: a prospective cohort study from a developing country. J Child Neurol. 2016;31:1622–7.
World Health Organization. WHO Consolidated Guidelines on Tuberculosis: Module 5: Management of Tuberculosis in Children and Adolescents: Web Annex 4. Summaries of Unpublished Studies. Available at: https://apps.who.int/iris/bitstream/handle/10665/352510/9789240046818-eng.pdf. Accessed on 22nd Dec 2022.
Author information
Authors and Affiliations
Contributions
KK and JLM reviewed the literature, analyzed the data, drafted, revised and reviewed the manuscript. JLM finalized the manuscript and will act as the guarantor for this mansucript.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, K., Mathew, J.L. World Health Organization Guideline on the Management of Tuberculosis in Children: Critical Appraisal, Concerns, and Caution. Indian J Pediatr 90, 811–816 (2023). https://doi.org/10.1007/s12098-023-04584-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-023-04584-y