Abstract
Lung carcinoids are rare tumors representing 1–2% of all invasive lung malignancies. They include typical and atypical carcinoids, whose distinction is made based on the mitotic index and presence or absence of necrosis. The 10-year overall survival for stage IV typical carcinoid is 47% and 18% for atypical carcinoid, reflecting the indolent growth of these tumors. There are limited approved treatment options for them and most of the evidence comes from retrospective analyses, single-arm trials, subgroup analysis of phase II/III trials for metastatic neuroendocrine tumors and extrapolation of data from phase III trials for gastroenteropancreatic neuroendocrine tumors. Management of metastatic lung carcinoids requires a multidisciplinary standardized approach in specialized centers. Treatment should have a dual objective, control of tumor growth and control of symptoms related to hypersecretion syndromes, aiming to improve quality of life and survival. In the continuum of treatment disease, locoregional treatment options need to be considered in parallel with systemic treatments. In this paper, we review the present treatment options and their rational and we give an insight into future alternatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung neuroendocrine neoplasms include a large spectrum of entities, from well-differentiated neuroendocrine tumors (NET) to poorly differentiated, high-grade neuroendocrine carcinomas (NEC). Lung NETs, also referred as lung carcinoids (LC), include typical and atypical carcinoids. The distinction between low-grade typical (TC) and intermediate-grade atypical carcinoid (AC), is based on the mitotic index and the presence of necrosis. Ki-67 index is not included in the WHO criteria, but it might be useful [1].
LC are rare tumors, representing 1–2% of all invasive lung malignancies, with an age-adjusted incidence rate of 0.2 to 2/100,000 population/year in US and Europe. However, over the last 30 years, there is a rising incidence, likely due to improved awareness and increased use of immunohistochemistry (IHC) [2, 3].
TC occur in the 4th to 6th decades of life and AC 1 decade later. The clinical presentation at diagnosis is non-specific tumor-related respiratory symptoms or incidental. A minority of cases present symptoms related to hormonal hypersecretion syndromes, such as carcinoid syndrome (CS), Cushing’s syndrome, and acromegaly [4, 5].
At diagnosis, TC are metastatic in up to 15% of cases, whereas ACs in up to one half of patients. The 10-year overall survival (OS) for stage IV TC is 47% and 18% for AC, reflecting the indolent growth of these tumors [7].
Due to its rarity, lung NETs are considered an orphan disease, with limited approved treatment options. Most of the evidence comes from retrospective analyses, single-arm trials, subgroup analysis of phase II/III trials of metastatic NET and data extrapolation from phase III trials dedicated to gastroenteropancreatic (GEP) NET. No specific phase III trial for LC has been published.
Here, we review the present treatment options for metastatic LC and future perspectives.
Present management of metastatic disease
Management of LC requires a multidisciplinary standardized approach in specialized centers. The multidisciplinary team should integrate medical oncologists, surgeons, pathologists, pulmonologists, radiologists, endocrinologists, and nuclear medicine specialists [4, 5].
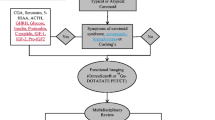
At the metastatic stage, patient’s performance status and comorbidities, WHO classification, chromogranin A levels, tumor burden and somatostatin receptor imaging uptake, as well as tumor growth kinetics and a hypersecretion syndrome presence, should be considered for adequate risk assessment and treatment decision.
Dual tracer PET/CT using 18F-FDG and 68 Ga-DOTATATE has been proposed to evaluate NET. This approach can better reflect tumor heterogeneity in metastatic sites and predict the clinical outcome. Specific biomarkers (5-HIAA, ACTH, cortisol, GHRH and IGF-1) are assessed depending on the presence of functional syndromes [4, 5].
Prolonged survival of most these patients, makes adjusted toxicity profile of therapeutic interventions critical. Treatment should have a dual objective, control of tumor growth and symptoms related to hypersecretion syndromes, aiming to improve quality of life (QoL) and survival. The best strategy, including sequencing, is unknown, due to the low number of dedicated trials and absence of predictors of response. Patients should be discussed frequently in multidisciplinary teams, to evaluate the possibility of locoregional treatments in the continuum of the disease. Re-biopsy should be considered, due to the possibility of dedifferentiation. Indeed, there is increasing evidence for a possible evolution to poorly differentiated NEC [8, 9].
Hormone-related symptom management
Considering one of the treatments goals, symptom control, the initial evaluation should look for hypersecretion syndromes. The most frequent is CS, and somatostatin analogs (SSA) are the first-line option, based on the results of PROMID (NCT00171873) and CLARINET (NCT00353496) trials [10, 11]. In patients with refractory CS, there is no consensus on the best strategy, due to the lack of specific trials. SSAs are also recommended for other functional syndromes such as acromegaly or hypercalcemia [4, 5]. For Cushing’s syndrome, metyrapone and/or ketoconazole are recommended as first-line therapy. If refractory, bilateral adrenalectomy should be considered [4, 5] .
Anti-tumor management
Treatment options vary from surveillance, locoregional or systemic treatments.
Surveillance, as a watchful follow-up, may be considered in asymptomatic TC and in low proliferative AC, after discussion with the patient, and as long as performing image evaluation every 3–6 months [5].
The locoregional treatments include surgery, interventional radiology or pulmonology, and radiotherapy.
Palliative surgery, radiofrequency ablation, cryoablation or endobronchial treatment of the primary tumor, are occasionally considered in cases of advanced disease, at risk of local events or for refractory CS [4, 5]. If pulmonary surgery with “curative” intent is considered, it should be reserved for patients with limited sites of metastatic disease, when radical excisional treatment is possible of all lesions [5]. Surgical resection of liver metastases may also be considered, with the purpose of symptom control or for debulking, when > 90% of tumor can be removed. Complete resection of liver metastases has shown to increased 5-year OS rates to > 70% [12]. Liver metastases are also potential targets for radiofrequency ablation or for selective embolization, with either bland particles or cytotoxics [4, 5].
Sometimes, locoregional treatments may represent the only anti-tumor strategy needed in patients with slowly progressive tumors, but the added value of combination of locoregional therapies, as an adjunct to surgery or systemic therapies, should be kept in mind in the continuum of disease [4, 5].
Systemic anti-tumor therapies for metastatic LC include SSAs, everolimus, chemotherapy (ChT), and peptide receptor radionuclide therapy (PRRT).
Octreotide and lanreotide are the two mostly used SSAs, based on the results of the randomized placebo-controlled phase III trials PROMID and CLARINET, that enrolled good prognostic or slowly progressive GEP-NETs. The PROMID randomly assigned 85 patients to octreotide long-acting release (LAR) versus (vs) placebo, showing an increase median progression-free survival (mPFS) of 14.3 vs 6 months [hazard ratio (HR) 0.34; 95% confidence interval (CI) 0.20–0.59] [10]. The CLARINET, randomly assigned 204 patients to lanreotide vs placebo, also demonstrated a significantly prolonged mPFS [not reached (NR) vs 18.0 months; HR 0.47; 95%CI 0.30–0.73] [11].
Data for use of SSAs in LCs are limited to multiple retrospective studies and a single prospective study. The placebo-controlled, randomized phase III trial, which evaluated lanreotide, in advanced LC was stopped for insufficient recruitment (SPINET trial NCT02683941). The study enrolled 77 patients, randomized 2:1, showed improve PFS (16.6 vs 13.6 moths, HR 0.90; 95%CI 0.46–1.88) and objective response rate (ORR) (14 vs 0%). Notably, patients with TC had an improved mPFS of 21 months, compared to 14.1 months in patients with AC [13].
Guidelines recommend that due to their excellent safety profile, SSAs should be considered the first-line treatment option for patients with advanced TC or slowly progressing somatostatin receptor (SSTR) positive AC [4,5,6]. After progression, treatment with SSAs should be discontinued, except in those patients with symptoms related to hypersecretion syndromes. In these cases, SSAs may be used in combination with any of the subsequent options, without any efficacy and safety concerns added, as shown in multiple trials, such as RADIANT-2 (NCT00412061), LUNA (NCT01563354) and ATLANT (NCT02698410) [14,15,16] .
The RADIANT-4 (NCT01524783) a randomized phase III trial, for well-differentiated, advanced, non-functional gastrointestinal or lung NETs, compared everolimus (205pts) to placebo (97pts). PFS showed significant improvement in the everolimus arm (11 vs 3.9 months; HR 0.48, p < 0.0001), while preserving the overall QoL. In the lung cohort (90 patients), PFS was also improved (HR 0.50) [17]. Nowadays, everolimus is the 1st line option for the majority ACs or following progression on SSAs for both TC and AC patients [4,5,6] .
LC generally have low proliferation index, so cytotoxic ChT often has limited effectiveness. However, ChT can be considered for AC rapidly progressive (RECIST progression in 3–6 months), high burden disease, Ki-67 > 15% and SSTR negative [18] .
The option usually relies on temozolomide-based ChT, based in the ECOG-ACRIN E2211 randomized phase II trial (NCT01824875), that compared temozolomide versus capecitabine/temozolomide (CAPTEM) in patients with advanced low-grade or intermediate-grade pancreatic NETs. The trial enrolled 144 patients and showed increase efficacy with the combination, with mPFS of 22.7 vs 14.4 months (HR0.58), and median OS 58.7 vs 53.8 months (HR 0.82). A secondary objective was to prospectively explore the predictive value of O-methylguanine methyltransferase (MGMT) status, by IHC and promoter methylation, for response to temozolomide-based ChT. The study found that MGMT deficiency was associated with response, since the RR in patients with low IHC expression was 52% vs 15% in those with high IHC expression and the RR for patients with promoter methylation was 85% vs 38% in those without [19]. However, further studies are needed to validate this data. Data with CAPTEM in LC are limited to small retrospective studies and the ORR reported is 21–30% [20, 21].
Small cell lung cancer regimens like platinum/etoposide, more widely used in NET G3, can also be considered in the same context, particularly in presence of a higher Ki-67 (> 20%) [18]. Small retrospective studies showed ORR of 23% with this option [22, 23].
Due to better tolerance and convenience, guidelines recommend ChT with CAPTEM as first-line option, and platinum-based ChT for second line in patients with progressive LC [4]. However, ChT treatment duration remains an open question.
Since somatostatin receptor type 2 (SSTR2) expression is the hallmark of well-differentiated NET, the advent of radiotheranostic treatments like PRRT, which combines diagnostic imaging with targeted therapies, has transformed NETs treatment. Based on the results of NETTER-1 trial (NCT01578239), lutetium [177Luoxodotreotide (177Lu-DOTATATE)] has been approved for treatment of unresectable or metastatic progressive well-differentiated (G1/G2), SSTR-positive GEP-NETs [24]. This trial randomized patients to either 177Lu-DOTATATE or high-dose octreotide LAR (60 mg). Patients who received 177Lu-DOTATATE had improved PFS (NR vs 8.4 months, HR 0.21; 95%CI 0.13–0.33) compared to those who received high-dose octreotide. In an updated report, the OS was 48.0 vs 36.3 months favoring lutetium, although not statistically significant, probably due to the 36% of crossover to the experimental arm [25]. Since its approval, 177Lu-DOTATATE has become widely accepted and recommended for LC. ESMO points it as an option in third or fourth lines, for patients SSTR positive, after progression on SSAs and everolimus. NCCN considers it a valid option in early lines [4, 6]. Once more, for the lung patient’s population, data are limited to retrospective studies. See Fig. 1 and Table 1.
Future perspectives
Antiangiogenics
Angiogenesis inhibitors have been explored in G1/G2 NET in multiple phase II/III trials; however, only sunitinib is currently registered in Europe and USA for pancreatic NET and surufatinib for extra-pancreatic NET in China and USA [26, 27] .
The SANET trial (NCT02588170), a randomized double-blind placebo-controlled phase III trial, comparing surufatinib vs placebo, enrolled 198 patients with well-differentiated extra-pancreatic NETs, after progression on no more than two systemic lines. The mPFS was favorable to surufatinib (9.2 vs 3.8 months, HR 0.33), and since it meets the predefined criteria at the interim analysis, the study was terminated early, as recommended by the independent data monitoring committee. One of the major limitations of the study is the inclusion of Chinese patients exclusively, so caution is need when extrapolating these results. [27] .
The CABINET trial (NCT03375320), a randomized, double-blind phase III study of cabozantinib vs placebo, in extra-pancreatic and pancreatic NETs, enrolled 290 patients. Patients were eligible if they had received at least one prior treatment including sunitinib, everolimus, or 177Lu-DOTATATE. The trial was stopped and unblinded earlier, by decision of the Safety Monitoring Board, due to the efficacy improvement with cabozantinib at an interim analysis. Patients in the placebo arm were allowed to crossover to cabozantinib, after unblinding. There was a significant improvement in PFS in both extra-pancreatic and pancreatic NETs. In extra-pancreatic NETs mPFS was 8.3 vs 3.2 months (HR 0.45) [28]. This puts cabozantinib as a potential new option for progressive GEP-NET, which might be extrapolated in the future to lung NETs.
Immune checkpoint inhibitors (ICI)
Up to date, results from clinical trials with monotherapy ICI in NET, have been disappointing. This may reflect trials’ design, since they included heterogeneous patients’ populations and sometimes, inclusively NEC, independently of the primary tumor origin and/or biomarkers. Data for LC are limited.
KEYNOTE-028 (NCT02054806), a phase I basket trial, evaluated pembrolizumab across 20 tumor cohorts, one of them dedicated to PD-L1 positive LC. This cohort included 25 patients, and the ORR was 12% (95%CI 2.5–31.2). The mPFS and OS was 5.6 (95%CI 3.5–10.7) and 21.1 months (95%CI 20.2-NR), respectively [29] .
KEYNOTE-158 (NCT02628067), a phase II multicohort trial, also evaluated pembrolizumab monotherapy, independently of PD-L1 expression. The cohort for well-differentiated NETs included 177 patients, 14 with LC. The ORR was 3.7% (95%CI 1.0–9.3) and the mPFS was 4.1 months (95%CI 3.5–5.4). No specific results per cohort were reported [30] .
Spartalizumab, an anti PD-1 monoclonal antibody, was evaluated in a phase II single-arm study for metastatic G1/G2 NET and poorly differentiated GEP-NEC. The study enrolled 95 patients in the NET group, 30 of them LC. The ORR was 7.4% (95%CI 3.0–14.6), but for LC, it was 20% and all responses were in AC patients [31] .
Due to limited efficacy with ICI monotherapy, combinations with anti CTLA-4, as well as with other agents have been explored. DART SWOG 1609 (NCT02834013), a phase II basket trial evaluated ipilimumab plus nivolumab in rare tumors, including a cohort for non-pancreatic NEN, with 32 patients, 19% LC. The ORR was 25% (95%CI 13–64), but 0% in low/intermediate-grade tumors (p = 0.004) [32] .
CA209-538 (NCT04969887), a phase II clinical trial, also evaluated ipilimumab plus nivolumab in rare tumors, including advanced NETs. Twenty-nine patients with advanced NETs were included, 39% LC. The ORR was 24%, but 0% in low-grade tumors. Three of nine patients (33%) with AC achieved an objective response, including two complete remissions [33] .
The combination of durvalumab and tremelimumab was evaluated in the DUNE phase II trial. Four different cohorts were established, one dedicated to LC (27 patients). In this cohort, ORR was 0%, with a clinical benefit rate of 7.4% [34].
Since NETs are generally classified as “cold” tumors, temozolomide was postulated to increase genomic instability and create neoantigens for immune recognition. The phase II trial NCT03728361, evaluated the combination of nivolumab plus temozolomide in patients with advanced NETs. This trial included 28 patients, 11 with LC. The ORR was 32.1% (95%CI 15.9–52.4), and the mPFS and OS was 11.1 (95%CI 3.0–29) and 32 months (95%CI 8.8-NR) respectively. Notably, in the lung cohort, there was a significantly improved ORR (64%; p = 0.020), with a PFS of 11.1 months (95%CI 3–29; p = 0.21) and an OS not reached at study completion (95%CI 8-NR; p = 0.602) [35]. The combination therapy may improve the sensitivity of the tumor microenvironment to ICI, adding other options of treatment.
Further prospective trials are needed, with better definition of inclusion criteria, namely origin of primary tumors. Furthermore, better biomarkers for patient selection to immunotherapy constitute an unmet need.
PRRT evolutions
There are ongoing attempts to improve PRRT. One of the strategies is the use of alpha emitting radionuclides, which have higher linear energy transfer, causing more damage to targeted cancer cells, and less damage to surrounding tissues. 213Bi-DOTATOC, 225Ac-DOTATATE, and 212Pb-DOTAMTATE are in evaluation. Another approach is the use of SSTR antagonists, such as 177Lu-satoreotide tetraxetan. Although they stay bound on the cell surface and are not internalized, they may have higher tumor uptake. Hence, it is expected to improve treatment responses, particularly when the density of SSTRs is low. Conjugation of DOTATATE to Evans blue analogs is also under investigation. Since Evans blue analogs reversibly bind to circulating serum albumin, this may extend half-life of DOTATATE in the blood. The limitation, however, has been the increase toxicity. Finally, there is emerging evidence with radioactive microspheres for liver metastases, expanding the options for locoregional treatments. Data for these new options are still premature, but promising [36,37,38].
Besides an increase of the therapeutic armamentarium, there is growing interest in challenging the present treatment algorithm. If PRRT is reserved to a more advanced line of treatment, it is expected to be less effective, due to the progressive loss of SSTR expression.
There is no consensus on the optimal timing to use PRRT in LC, due to lack of data. For GEP-NETs however, there are data that might change clinical practice. The NETTER-2 (NCT03972488) evaluated 177Lu-DOTATATE plus octreotide LAR (30 mg) vs octreotide LAR (60 mg) as first-line treatment in patients with G2/G3 advanced GEP-NETs. The trial included 226 patients, showed an increase in mPFS (22.8 vs 8.5 months (HR0.28)) and in ORR (43.0 vs 9.3%) with the combination [39] .
In the same setting, results from COMPOSE (NCT04919226) and COMPETE (NCT03049189) phase III trials on GET-NET are pending, that could validate an earlier treatment with 177Lu-edotreotide PRRT [40, 41]. These are all trials for GEP-NET, but if successful, results might be extrapolated to LC, like it happened with NETTER-1. However, we might have a better insight with the ongoing ALLIANCE (A021901) randomized phase II trial (NCT04665739) comparing 177Lu-DOTATATE to everolimus, in patients with advanced LC [42].
Finally, efforts are being made to create clinical tools to assist treatment decisions, such as a PRRT Predictive Quotient and the NETest [43, 44] .
Others
There has been an increase interest in SSTR2-targeted treatments, although most of them are still in early phase of development. Some examples are PEN-221 (NCT02936323) and tidutamab, already evaluated in dose escalation phase I trials [36] .
Epigenetic drugs are also being explored in this context, since whole-genome sequencing of pancreatic NETs has revealed inactivation of genes involved in chromatin remodeling and histone modifications. For now, data are limited to in vitro studies or in vivo xenograft models [36] .
Conclusion
Management of LC requires a multidisciplinary approach in specialized centers. At the metastatic stage, multiple factors should be considered for adequate risk assessment and treatment decision. Treatment should have a dual objective—control of tumor growth and symptoms—aiming to improve QoL and survival, in a usually indolent disease. The therapeutic approaches should integrate locoregional and systemic treatments in the continuum of the disease. Data on LC remain poor. Future trials should follow the recommendations of neuroendocrine task forces, namely, conducting whenever possible trials separately for bronchial, midgut, and pancreatic NETs, and if not feasible, stratifying patients according to site of origin, tumor grade and differentiation.
References
WHO. Classification of Tumours. In: Thoracic Tumors. Lyon: IARC Press; 2021.
Dasari A, Shen C, Halperin D, Bo Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–42. https://doi.org/10.1001/jamaoncol.2017.0589.
Korse CM, Taal BG, van Velthuysen MLF, Visser O. Incidence and survival of neuroendocrine tumours in the Netherlands according to histological grade: experience of two decades of cancer registry. Eur J Cancer. 2013;49(8):1975–83. https://doi.org/10.1016/j.ejca.2012.12.022.
Baudin E, Caplin M, Garcia-Carbonero R, Fazio N, Ferolla P, Filosso PL, Frilling A, de Herder WW, Hörsch D, Knigge U, Korse CM, Lim E, Lombard-Bohas C, Pavel M, Scoazec JY, Sundin A, Berruti A. Lung and thymic carcinoids: ESMO clinical practice guidelines for diagnosis, treatment, and follow-up. Ann Oncol. 2021;32(4):439–51. https://doi.org/10.1016/j.annonc.2021.01.003.
Caplin ME, Baudin E, Ferolla P, Filosso P, Garcia-Yuste M, Lim E, Oberg K, Pelosi G, Perren A, Rossi RE, Travis WD, Bartsch D, Capdevila J, Costa F, Cwikla J, de Herder W, Fave GD, Eriksson B, Falconi M, Ferone D, Gross D, Grossman A, Ito T, Jensen R, Kaltsas G, Kelestimur F, Kianmanesh R, Knigge U, Kos-Kudla B, Krenning E, Mitry E, Nicolson M, O’Connor J, O’Toole D, Pape U-F, Pavel M, Ramage J, Raymond E, Rindi G, Rockall A, Ruszniewski P, Salazar R, Scarpa A, Sedlackova E, Sundin A, Toumpanakis C, Vullierme M-P, Weber W, Wiedenmann B, Zheng-Pei Z. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26(8):1604–20. https://doi.org/10.1093/annonc/mdv041.
NCCN. 2023. Clinical practice guidelines neuroendocrine and adrenal tumors Version 1
Yoon JY, Sigel K, Martin J, Robyn Jordan R, Beasley MB, Smith C, Kaufman A, Juan Wisnivesky J, Kim MK. Evaluation of the prognostic significance of TNM staging guidelines in lung carcinois tumors. J Thoracic Oncol. 2019;14:184–92. https://doi.org/10.1016/j.jtho.2018.10.166.
Pelosi G, Bianchi F, Dama E, Simbolo M, Mafficini A, Sonzogni A, Pilotto S, Harari S, PapottiM VM, Fontanini G, Mastracci L, Albini A, Bria E, Calabrese SA. Most high-grade neuroendocrine tumours of the lung are likely to secondarily develop from pre-existing carcinoids: innovative findings skipping the current pathogenesis paradigm. Virchows Arch. 2018;472:567–77. https://doi.org/10.1007/s00428-018-2307-3.
Pelosi G, Sonzogni A, Harari S, Albini A, Bresaola E, Marchiò C, Massa F, Righi G, Papanikolaou N, Vijayvergia N, Calabrese F, Papotti M. Classification of pulmonary neuroendocrine tumors: new insights. Transl Lung Cancer Res. 2017;6:513–29. https://doi.org/10.21037/tlcr.2017.09.04.
Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Blaker M, Harder J, Arnold C, Gress TM, Arnold R. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656–63. https://doi.org/10.1200/JCO.2009.22.8510.
Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–33. https://doi.org/10.1056/NEJMoa1316158.
Glazer ES, Tseng JF, Al-Refaie W, Solorzano CS, Liu P, Willborn KA, Abdalla EK, Vauthey JN, Curley SA. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB (Oxford). 2010;12:427–33. https://doi.org/10.1111/j.1477-2574.2010.00198.x.
Baudin E, Horsch D, Singh S, Caplin ME, Ferone D, Wolin EM, Capdevila J, Buikhuisen WA, Raderer M, Dansin E, Grohe C, Houchard A, Truong Thanh X-M, Reidy-Lagunes D. Lanreotide autogel/depot (LAN) in patients with advanced bronchopulmonary (BP) neuroendocrine tumors (NETs): results from the phase III SPINET study. Ann Oncol. 2021. https://doi.org/10.1016/j.annonc.2021.08.178.
Fazio N, Granberg D, Grossman A, Saletan S, Klimovsky J, Panneerselvam A, Wolin EM. Everolimus plus octreotide long-acting repeatable in patients with advanced lung neuroendocrine tumors: analysis of the phase 3, randomized, placebo-controlled RADIANT-2 study. Chest. 2013;143(4):955–62. https://doi.org/10.1378/chest.12-1108.
Ferolla P, Brizzi MP, Meyer T, Mansoor W, Mazieres J, Do Cao C, Léna H, Berruti A, Damiano V, Buikhuisen W, Grønbæk H, Lombard-Bohas C, Grohé C, Minotti V, Tiseo M, De Castro J, Reed N, Gislimberti G, Singh N, Stankovic M, Öberg K, Baudin E. Efficacy and safety of long-acting pasireotide or everolimus alone or in combination in patients with advanced carcinoids of the lung and thymus (LUNA): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2017;18(12):1652–64. https://doi.org/10.1016/S1470-2045(17)30681-2.
Ferolla P, Berruti A, Spada F, Brizzi MP, Ibrahim T, Marconcini R, Giuffrida D, Amoroso V, La Salvia A, Vaccaro V, Faggiano A, Colao A, Volante M, Ghizzoni S, Mazzanti P, Houchard A, Fazio N. Efficacy and safety of lanreotide autogel and temozolomide combination therapy in progressive thoracic neuroendocrine tumors (Carcinoid): results from the phase 2 ATLANT study. Neuroendocrinology. 2023;113(3):332–42. https://doi.org/10.1159/000526811.
Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, Pacaud LB, Rouyrre N, Sachs C, Valle JW, Fave GD, Van Cutsem E, Tesselaar M, Shimada Y, Oh DY, Strosberg J, Kulke MH, Pavel ME. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(10022):968–77. https://doi.org/10.1016/S0140-6736(15)00817-X.
Pavel M, O’Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R, Krenning E, Knigge U, Salazar R, Pape U-F, Öberg K. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. 2016. https://doi.org/10.1159/000443167.
Kunz PL, Graham NT, Catalano PJ, Nimeiri HS, Fisher GA, Longacre TA, Suarez CJ, Martin BA, Yao JC, Kulke MH, Hendifar AE, Shanks JC, Shah MH, Zalupski MM, Schmulbach EL, Reidy-Lagunes DL, Strosberg JR, O’Dwyer PJ, Benson AB 3rd. Randomized Study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors (ECOG-ACRIN E2211). J Clin Oncol. 2023;41(7):1359–69. https://doi.org/10.1200/JCO.22.01013.
Thomas K, Voros BA, Meadows-Taylor M, Smeltzer MP, Griffin R, Boudreaux JP, Thiagarajan R, Woltering EA, Ramirez RA. Outcomes of capecitabine and temozolomide (CAPTEM) in advanced neuroendocrine neoplasms (NENs). Cancers. 2020;12(1):206. https://doi.org/10.3390/cancers12010206.
Al-Toubah T, Morse B, Strosberg J. Capecitabine and temozolomide in advanced lung neuroendocrine neoplasms. Oncologist. 2020;25:48–52. https://doi.org/10.1634/theoncologist.2019-0361.
Forde PM, Hooker CM, Boikos SA, Petrini I, Giaccone G, Rudin C, Yang SC, Illei PB, Hann CL, Ettinger DS, et al. Systemic therapy, clinical outcomes, and overall survival in locally advanced or metastatic pulmonary carcinoid: A brief report. J Thorac Oncol. 2014;9:414–8. https://doi.org/10.1097/JTO.0000000000000065.
Chong CR, Wirth LJ, Nishino M, Chen AB, Sholl LM, Kulke MH, McNamee CJ, Jänne PA, Johnson BE. Chemotherapy for locally advanced and metastatic pulmonary carcinoid tumors. Lung Cancer. 2014;86:241–6. https://doi.org/10.1016/j.lungcan.2014.08.012.
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O’Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Sierra ML, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E. NETTER-1 trial investigators. phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017. https://doi.org/10.1056/NEJMoa1607427.
Strosberg JR, Caplin ME, Kunz PL, Ruszniewski PB, Bodei L, Hendifar A, Mittra E, Wolin EM, Yao JC, Pavel ME, Grande E, Van Cutsem E, Seregni E, Duarte H, Gericke G, Bartalotta A, Mariani MF, Demange A, Mutevelic S, Krenning EP. NETTER-1 investigators. (177) Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomized, controlled, phase 3 trial. Lancet Oncol. 2021. https://doi.org/10.1016/S1470-2045(21)00572-6.
Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011. https://doi.org/10.1056/NEJMoa1003825.
Jianming Xu, Shen L, Zhou Z, Li J, Bai C, Chi Y, Li Z, Nong Xu, Li E, Liu T, Bai Y, Yuan Y, Li X, Wang X, Chen J, Ying J, Xianjun Yu, Qin S, Yuan X, Zhang T, Deng Y, Xiu D, Cheng Y, Min Tao Ru, Jia WW, Li J, Fan S, Peng M, Weiguo Su. Surufatinib in advanced extra-pancreatic neuroendocrine tumours (SANET-ep): a randomized, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(11):1500–12. https://doi.org/10.1016/S1470-2045(20)30493-9.
Chan J, Geyer S, Ou F-S, Knopp M, Behr S, Zemla T, Acoba J, Shergill A, Wolin EM, Halfdanarson TR, Trikalinos N, Konda B, Vijayvergia N, Dasari NA, Strosberg J, Kohn EC, Kulke MH, O’Reilly EM, Meyerhardt JA. Alliance A021602: phase III, double-blinded study of cabozantinib versus placebo for advanced neuroendocrine tumors (NET) after progression on prior therapy (CABINET). Ann Oncol. 2023;34(52):S1292.
Mehnert JM, Bergsland E, O’Neil BH, Santoro A, Schellens JHM, Cohen RB, et al. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: results from the KEYNOTE-028 study. Cancer. 2020;126(13):3021–30. https://doi.org/10.1002/cncr.32883.
Strosberg J, Mizuno N, Doi T, Grande E, Delord JP, Shapira Frommer R, et al. Efficacy and safety of pembrolizumab in previously treated advanced neuroendocrine tumors: results from the phase II KEYNOTE-158 study. Clin Cancer Res. 2020;26(9):2124–30. https://doi.org/10.1158/1078-0432.CCR-19-3014.
Yao JC, Strosberg J, Fazio N, Pavel ME, Ruszniewski P, Bergsland E, Li D, Tafuto S, Raj N, Campana D, Hijioka S, Raderer M, Guimbaud R, Gajate P, Pusceddu S, Reising A, Degtyarev E, Mookerjee B, Aimone P, Singh S. Activity & safety of spartalizumab (PDR001) in patients (pts) with advanced neuroendocrine tumors (NET) of pancreatic (Pan), gastrointestinal (GI), or thoracic (T) origin, & gastroenteropancreatic neuroendocrine carcinoma (GEP NEC) who have progressed on prior treatment (Tx). Ann Oncol. 2018;29(8):S1308O.
Patel SP, Othus M, Chae YK, Giles FJ, Hansel DE, Singh PP, Fontaine A, Shah MH, Kasi A, Baghdadi TA, Matrana M, Gatalica Z, Korn WM, Hayward J, McLeod C, Chen HX, Sharon E, Mayerson E, Ryan CW, Plets M, Blanke CD, Kurzrock R. A phase ii basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART SWOG 1609) in patients with nonpancreatic neuroendocrine tumors. Clin Cancer Res. 2020;26(10):2290–6. https://doi.org/10.1158/1078-0432.CCR-19-3356.
Klein O, Kee D, Markman B, Michael M, Underhill C, Carlino MS, Jackett L, Lun C, Scott C, Nagrial A, Behren A, So JY, Palmer J, Cebon J. Immunotherapy of ipilimumab and nivolumab in patients with advanced neuroendocrine tumors: a subgroup analysis of the CA209–538 clinical trial for rare cancers. Clin Cancer Res. 2020;26(17):4454–9. https://doi.org/10.1158/1078-0432.CCR-20-0621.
Capdevila J, Hernando J, Teule A, Lopez C, Garcia-Carbonero R, Benavent M, Custodio A, Garcia-Alvarez A, Cubillo A, Alonso V, Carmona-Bayonas A, Alonso-Gordoa T, Crespo G, Jimenez-Fonseca P, Blanco M, Viudez A, La Casta A, Sevilla I, Segura A, Llanos M, Landolfi S, Nuciforo P, Manzano JL. Durvalumab plus tremelimumab for the treatment of advanced neuroendocrine neoplasms of gastroenteropancreatic and lung origin. Nat Commun. 2023;14(1):2973. https://doi.org/10.1038/s41467-023-38611-5.
Owen DH, Benner B, Wei L, Sukrithan V, Goyal A, Zhou Y, Pilcher C, Suffren SA, Christenson G, Curtis N, Jukich M, Schwarz E, Savardekar H, Norman R, Ferguson S, Kleiber B, Wesolowski R, Carson WE, Otterson GA, Verschraegen CF, Shah MH, Konda B. A phase II clinical trial o nivolumab and temozolomide for neuroendocrine neoplasms. Clin Cancer Res. 2023;29(4):731–41. https://doi.org/10.1158/1078-0432.CCR-22-1552.
Garcia-Alvarez A, Cubero JH, Capdevila J. Drug development in neuroendocrine tumors: what is on the horizon? Curr Treat Options in Oncol. 2021;22:43. https://doi.org/10.1007/s11864-021-00834-3.
Rutherford M, Wheless M, Thomas K, Ramirez RA. Current and emerging strategies for the management of advanced/ metastatic lung neuroendocrine tumors. Curr Probl Cancer. 2024. https://doi.org/10.1016/j.currproblcancer.2024.101061.
Mulvey CK. Emerging precision medicine approaches for lung neuroendocrine tumors. Cancers. 2023;15(23):5575. https://doi.org/10.3390/cancers15235575.
Singh S, Halperin DM, Myrehaug S, Herrmann K, Pavel M, Kunz PL, Chasen B, Capdevila J, Tafuto S, Oh DY, Yoo C, Falk S, Halfdanarson TR, Folitar I, Zhang Y, Santoro P, Aimone P, Herder WW, Ferone D. (177Lu) Lu-DOTA-TATE in newly diagnosed patients with advanced grade 2 and grade 3, well-differentiated gastroenteropancreatic neuroendocrine tumors: primary analysis of the phase 3 randomized NETTER-2 study. Gastroenteropancreatic Neuroendocr Tumors. 2024. https://doi.org/10.1200/JCO.2024.42.3_suppl.LBA588.
Halfdanarson TR, Reidy DL, Vijayvergia N, Halperin DM, Goldstein G, Kong G, Michael M, Leyden S, Grozinsky-Glasberg S, Sorbye H, Oberg KE, Sierras C, Harris P. Pivotal phase III COMPOSE trial will compare 177Lu-edotreotide with best standard of care for well-differentiated aggressive grade 2 and grade 3 gastroenteropancreatic neuroendocrine tumors. J Clinical Oncol. 2022. https://doi.org/10.1200/JCO.2022.40.4_suppl.TPS514.
Wahba MM, Strosberg J, Avram A, Aparici CM. COMPETE phase III trial-peptide receptor radionuclide therapy (PRRT) with 177Lu-edotreotide vs everolimus in progressive GEP-NET. Cancer Res. 2021. https://doi.org/10.1158/1538-7445.AM2021-CT254.
Testing lutetium lu 177 dotatate in patients with somatostatin receptor positive advanced bronchial neuroendocrine tumors. https://classic.clinicaltrials.gov/ct2/show/NTC04665739
Roll W, Weckesser M, Seifert R, Bodei L, Rahbar K. Imaging and liquid biopsy in the prediction and evaluation of response to PRRT in neuroendocrine tumors: implications for patient management. Eur J Nucl Med Mol Imaging. 2021;202(48):4016–27. https://doi.org/10.1007/s00259-021-05359-3.
Modlin IM, Kidd M, Malczewska A, Drozdov I, Bodei L, Matar S, Chung KM. The NETest: the clinical utility of multigene blood analysis in the diagnosis and management of neuroendocrine tumors. Endocrinol Metab Clin North Am. 2018;47(3):485–504. https://doi.org/10.1016/j.ecl.2018.05.002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Data availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rodrigues, A., Henrique, R., Jerónimo, C. et al. Management of typical and atypical metastatic lung carcinoids: present and future perspectives. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03607-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03607-0