Abstract
Background
Few studies have been designed to predict the survival of Chinese patients initially diagnosed with metastatic gastric cancer (mGC). Therefore, the objective of this study was to construct and validate a new nomogram model to predict cancer-specific survival (CSS) in Chinese patients.
Methods
We collected 328 patients with mGC from Northern Jiangsu People’s Hospital as the training cohort and 60 patients from Xinyuan County People’s Hospital as the external validation cohort. Multivariate Cox regression was used to identify risk factors, and a nomogram was created to predict CSS. The predictive performance of the nomogram was evaluated using the consistency index (C-index), the calibration curve, and the decision curve analysis (DCA) in the training cohort and the validation cohort.
Results
Multivariate Cox regression identified differentiation grade (P < 0.001), T-stage (P < 0.05), N-stage (P < 0.001), surgery (P < 0.05), and chemotherapy (P < 0.001) as independent predictors of CSS. Nomogram of chemotherapy regimens and cycles was also designed by us for the prediction of mGC. Thus, these factors are integrated into the nomogram model: the C-index value was 0.72 (95% CI 0.70–0.85) for the nomogram model and 0.82 (95% CI 0.79–0.89) and 0.73 (95% CI 0.70–0.86) for the internal and external validation cohorts, respectively. Calibration curves and DCA also demonstrated adequate fit and ideal net benefit in prediction and clinical applications.
Conclusions
We established a practical nomogram to predict CSS in Chinese patients initially diagnosed with mGC. Nomograms can be used to individualize survival predictions and guide clinicians in making therapeutic decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Although the incidence of gastric cancer (GC) is decreasing, it remains the fourth leading cause of cancer-related deaths. Globally, more than 1,000,000 new cases are diagnosed each year, and the number of deaths from GC is about 768,800 each year; in China, there are approximately 477,800 new cases and 373,200 deaths each year [1,2,3]. The 5-year survival rate for patients with GC with distant metastasis is less than 5% and the median survival time is 11–18 months [4, 5]. Treatment of GC depends mainly on the stage of the tumor, and for non-metastatic gastric cancer, attention should be paid to supplementing adjuvant chemotherapy or radiation therapy while applying surgical treatment, and a combination of therapeutic methods is often adopted for mGC [6,7,8].
Despite the great progress in lymph node dissection, neoadjuvant chemoradiotherapy, targeted therapy, and immunotherapy, the rate of early GC diagnosis is very low due to the lack of typical clinical symptoms in early GC, and most patients are already in an advanced stage at the time of diagnosis, especially in China, where almost 80% of the patients with GC are diagnosed with advanced or metastatic disease [9,10,11]. The assessment of mortality risk in patients with GC is still based on the TNM classification system of the American Joint Committee on Cancer (AJCC) [12]. In particular, there is significant heterogeneity among GC patients in terms of demographic and clinicopathological characteristics, such as age, gender, body mass index, tumor size, differentiation grade, metastatic organ sites and number, as well as applied treatment regimens, and clinicopathological characteristics, which may affect the prognosis of the patients; however, they are not reflected in the TNM classification system. Good clinical decision-making, clinical trial design, and an accurate prognosis are needed to predict high-risk patient groups. The use of nomograms is of great clinical help, and there are many nomograms that have been produced for the diagnosis of the disease, and perform better than the TNM classification system of the AJCC [13,14,15]. Although there have been many nomograms based on different databases to predict the survival of individuals with GC, few studies have been designed to predict the survival of Chinese patients initially diagnosed with mGC [16,17,18,19,20]. Therefore, the objective of this study was to construct and validate a new nomogram model to predict CSS in Chinese patients with mGC.
Materials and methods
Patients and data collection
We collected 328 patients with mGC diagnosed for the first time at Northern Jiangsu People’s Hospital from January 2012 to December 2022 as a training cohort. Based on the same criteria, 60 patients at Xinyuan County People’s Hospital from January 2018 to December 2022 were collected as an external validation cohort.
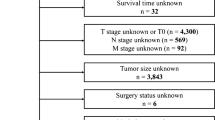
Inclusion criteria for this study: (1) histological diagnosis of gastric adenocarcinoma; (2) age ≥ 18 years; (3) inclusion of patients initially diagnosed with distant organ metastasis and/or lymph node metastasis based on the AJCC staging system; (4) availability of complete hospitalization and follow-up data; and (5) the ultimate cause of death was sex-related death related to gastric cancer. Exclusion criteria: (1) age < 18 years; (2) comorbidity with other tumors; and (3) lack of or incomplete clinical data, including information on the depth of tumor infiltration (T-stage) lymph node metastasis (N-stage) and distant metastasis (M-stage), and distant organ metastases of gastric cancer (Fig. 1). The TNM staging was re-staged according to the AJCC 8th edition staging system.
Finally, the collected 328 patients were randomly divided into two cohorts according to 7:3, one group was a modeling cohort of 229 patients for studying CSS and the construction of nomogram models, and the other group was an internal validation cohort of 99 patients to validate the nomogram models. The 60 patients collected from Xinyuan County People’s Hospital were used as the external validation sequence. All gastric cancers were initially diagnosed by computed tomography or positron emission tomography computed tomography with at least one distant metastasis, including the peritoneum, liver, lung, bone, brain, kidney, pancreas, spleen and ovary. The ethics committees of the Northern Jiangsu People’s Hospital and the Xinyuan County People’s Hospital had approved the study.
Follow-up and outcome
Follow-up methods included medical records and telephone follow-up. Observations were made every 1 month for the first 6 months after treatment, every 3 months for the next 6 months, every 6 months for 2–3 years, and annually thereafter. Each follow-up visit included physical examination, laboratory tests, and chest/abdominal/pelvic enhancement CT. The last follow-up date was December 2022.
Endpoint definition
GC-specific death was defined as death from GC as the underlying cause according to our data, and the endpoint of the current study was CSS, which was the interval between the initial diagnosis of GC and the occurrence of GC-specific death.
Statistical analysis and nomogram construction
We use X-tile software to determine the optimal cutoff value for the stratification of continuous variables, such as age, body mass index (BMI), and tumor size, the risk value that would stratify cancer-related deaths. Taking into account the significant effect of age on survival risk, age was divided into two groups based on the optimal cutoff values calculated by the X-tile software with a cutoff of 75 years. Body mass index (BMI) was divided into two groups with a cutoff of 19.5 kg/m2. Tumor size was divided into 3 groups with 6.0 cm and 8.0 cm as cutoffs, respectively (Fig. 2). Data were analyzed using SPSS statistical software (version 26.0, IBM, USA), and clinical characteristics were described as percentages, medians, and interquartiles (IQR). Comparisons of categorical variables were made using the Chi-square test or Fisher’s exact test. A two-tailed P < 0.05 was considered statistically significant. Significant variables from the univariate analysis were included in the multivariate Cox regression to identify independent factors. Survival curves of the independent factors were plotted using Kaplan–Meier analysis (Fig. 3). Based on the final multivariate Cox regression, the nomogram was constructed using the rms and survival packages in R language (version 4.3.2, http://www.r-project.org).
Survival curves for each clinical variable after multifactorial analysis. A Survival curves for degree of tumor differentiation. B Survival curves for T-stage (according to the AJCC 8th edition staging system). C Survival curves for N-stage (according to the AJCC 8th edition staging system). D Survival curves for surgery. E Survival curves for chemotherapy
The training cohort was randomly divided into two groups by 7:3 using R. The modeling group was used to build the nomogram model, the internal and external validation groups were used to validate the model. Consistency index (C-index), calibration curve and decision curve analysis (DCA) were used to assess the predictive efficacy of the nomogram. C-index was used to assess the predictive accuracy and discriminative power of the nomogram model. The calibration curve is used to assess the calibration of the model. DCA was used to evaluate clinical utility.
Results
Baseline characteristics
According to our inclusion and exclusion criteria (Fig. 1), a total of 328 patients with initial diagnosis of mGC from 2012 to 2022 from the Northern Jiangsu People’s Hospital were finally included in this study. All cases were diagnosed with mGC at the time of initial diagnosis. The mean age of the patients was 63.37 ± 10.175 years. 68.90% of these patients (N = 226) were male. The most common site of metastasis was the liver (51.52%) followed by the peritoneum (46.65%), lung (7.62%) and bone (7.01%). Table 1 shows the characteristics of the patients. All eligible cases were randomly assigned to the training cohort (229, 70%) or the internal validation cohort (99, 30%) to create and validate the prognostic nomogram; the cohort from Xinyuan County People’s Hospital was used for external validation. The median CSS was 9.4 months [95% CI 8.03–10.77] and 11.6 months [95% CI 8.84–14.33] in the training cohort and internal validation cohort, respectively, and the median CSS was 11.7 months [95% CI 9.18–14.32] for the external cohort, and Table 2 summarizes the characteristics of the patients in the two cohorts. The treatment strategy for all patients was developed by a multidisciplinary team of surgeons, medical oncologists, and radiologists from the Northern Jiangsu People’s Hospital. Clinical staging was further confirmed by experienced radiologists, and chemotherapy regimens used for patients with metastatic gastric cancer mainly included mFLOT and FOLFOX (or its derivatives, such as SOX or XELOX). (1) FOLFOX: Oxaliplatin 85 mg/m2 + Fluorouracil 2800 mg/m2 continuous intravenous injection over 48 h; every 2 weeks; (2) SOX: Oxaliplatin 130 mg/m2 intravenous injection + Tegafur Gimeracil Oteracil Potassium Capsule 40–60 mg bid D1–D14; every 3 weeks; (3) XELOX: Oxaliplatin 130 mg/m2 + Capecitabine 1000 mg/m2 bid D1–D14; every 3 weeks; and (4) mFLOT: Docetaxel 50–60 mg/m2 + Oxaliplatin 85 mg/m2 + Fluorouracil 2800 mg/m2 intravenous injection over 48 h; every 2 weeks (Table 3).
Independent prognostic factors in the training cohort
Univariate analysis showed that differentiation grade, clinical T stage, N stage, liver metastasis, peritoneal metastasis, surgery, chemotherapy, and immunotherapy were associated with the prognosis of the patient. Factors with a P value < 0.05 in the univariate analysis were included in the multivariate Cox regression model. Multivariate analysis identified differentiation grade (P < 0.001), T-stage (P < 0.05), N-stage (P < 0.001), surgery (P < 0.05), and chemotherapy (P < 0.001) as independent predictors of CSS, and these were included in the prediction model (Table 4).
Prognostic nomogram for CSS
The predictive model was in the form of a nomogram. Significant independent factors such as differentiation classification, T stage, N stage, surgery, and chemotherapy were used to create the nomogram (Fig. 4). Chemotherapy contributed the most to the prognosis, followed by differentiation grading. The T/N stage, and surgery had a moderate effect on survival. So we created another nomogram about chemotherapy regimens and cycles, which was used to predict the predictive ability of mGC under different chemotherapy regimens and cycles (Fig. 5). Each variable was assigned a score from 0 to 10, which is shown at the top of the column chart; after calculating the total score and positioning it on a scale of total score, a straight line was plotted at different times relative to the date of diagnosis to determine the estimated survival rate.
Calibration and validation of the nomogram
For the training cohort, the consistency index value was 0.72 (95% CI 0.70–0.85), reflecting the good discriminatory power of the model. The calibration curves also show good agreement in terms of observations and nomogram predictions regarding CSS at 6 months, 1 year, and 2 years (Fig. 6A–C). For the internal validation cohort, with a c-index value of 0.82 (95% CI 0.79–0.89), and the calibration plots showed acceptable agreement between the predictions of the nomogram and observations with respect to CSS at 6 months, 1-year, and 2-year CSS (Fig. 6D–F). For the external validation cohort, the value of the c-index was 0.73 (95% CI 0.70–0.86) (Fig. 6G–I). CSS based on DCA, the nomogram model showed clinical utility at different time points. Compared to the traditional AJCC staging system, it has been confirmed that clinical net benefits for nomogram model at the time endpoint of 6-month, 1-year, and 2-year CSS were better, with a wide range of threshold probabilities in both training (Fig. 7A–C). In Fig. 7A–C, the green line represents the net benefit line for “Treat None”, which assumes that no patients are treated and the net benefit is zero. The red curve represents the net benefit of “Treat All”, which assumes that all patients are treated, and shows the net benefit in this extreme case. The blue curve represents the net benefit of the nomogram. As can be seen in Fig. 7A–C, the overall net benefit of the nomogram is superior than all of the patients’ dead scheme or none of the patients’ dead scheme over the entire range (0–1).
Calibration of the nomogram at 6 months, 1 year, and 2 years in the training, internal validation, and external validation cohorts. The nomogram-predicted CSS is plotted on the x-axis and the actual CSS is plotted on the y-axis. The 45° line indicates a perfect prediction model. The 6-month, 1-year, and 2-year CSS rates of the (A–C) training, (D–F) internal validation, and (G–I) external validation cohorts
Decision curve analysis of column line graphs for predicting CSS. Based on data from the primary cohort, decision curve analysis was used to determine the clinical utility of column line plots for predicting CSS at the 6-month (A), 1-year (B), and 2-year (C) time points. The horizontal green line corresponds to no cancer-specific deaths, and the solid red line assumes that all patients die of cancer. The blue line represents the net benefit of using the column chart
Discussion
Although some nomograms have been established to predict the prognosis of gastric cancer patients, there are few prediction models for mGC patients of Chinese. We established and validated a nomogram to predict CSS in patients with mGC based on their clinicopathological characteristics and treatment regimen. The guidelines of the National Comprehensive Cancer Network recommend chemotherapy as the first-line treatment for patients with mGC, advanced gastric cancer, or recurrent gastric cancer. Chemotherapy can prolong OS (overall survival) and improve quality of life [21]. A meta-analysis suggests that chemotherapy prolongs the OS of patients with mGC by approximately 6.7 months compared with best supportive care (BSC) [22]. A phase III Randomized Clinical Trial suggests that combination therapy was associated with numerically improved overall survival (OS), although statistically insignificant, and a significant progression-free survival (PFS) benefit compared with monotherapy [23]. Our nomogram also showed better CSS with multidrug combination therapy than with monotherapy. Another meta-analysis suggests that S-1-based combination therapy is superior to S-1 monotherapy in terms of overall survival (OS), progression-free survival (PFS), and overall response rate (ORR) [24]. Chemotherapy cycles show in our Nomogram that a long number of cycles is superior to a short number of cycles for metastatic gastric cancer in terms of CSS. A pooled analysis of the OGSG0604 and OGSG1002 trials suggests that the long cycles (≥ 6) of Docetaxel plus S − 1 capsule (DS) therapy may prolong prognosis [25]. This is also consistent with the Nomogram results we produced.
Currently, the preoperative clinical staging of gastric cancer patients is based on the classification of TNM, which is the most widely used gastric cancer staging system for staging gastric cancer patients, evaluating prognosis, and formulating treatment plans [26]. In the primary lesion of gastric cancer, the aggressiveness of tumor cells increases with increasing invasion depth, and then the phenomenon of invasion of vascular lymphatic vessels may appear [27, 28]. Studies have shown that tumor invasion depth and lymph node metastasis are independent prognostic factors in determining survival of gastric cancer patients [29], and our study found the same results. Although TNM staging is generally recognized, there are certain limitations. Gastric cancer is highly heterogeneous and prone to recurrence and metastasis, and the TNM staging does not take into account these biological characteristics of gastric cancer, which may cause bias in the assessment of the disease, resulting in different efficacy after treatment [30]. Chmelo et al. believe that the detection of lymphatic, venous and nerve invasion can help improve the precision of the prognosis of gastric cancer patients, and can also help stratify patients with high risk of recurrence and better guide the decision-making process of adjuvant chemotherapy [31, 32]. Although the T and N stages extracted from our data in this study belong to the 8th edition of the AJCC pathological stage of tumors, because they are both stages M1, and the definitions of tumor invasion depth and regional lymph node metastasis have hardly changed after comparing the 7th and 8th editions of TNM stages, the 8th edition TNM stage used in this study will not have a significant impact on the results and limit the use of the constructed prediction model.
At present, the effect of surgery on the prognosis of patients with mGC has been controversial [33, 34]. However, most patients with mGC are generally unable to undergo radical surgery due to non-curative factors, such as distant metastasis, and only undergo non-curative surgeries, such as palliative surgery and dose reduction surgery [35]. Previous studies have suggested that surgery can improve the prognosis of patients with mGC [36,37,38,39,40], and resection of tumor lesions resetion can reduce the tumor burden of patients, prevent or alleviate the occurrence of some complications caused by tumors, and improve the tolerance of patients to chemotherapy to some extent. However, surgical treatment may delay the time of chemotherapy, thereby reducing the effect of chemotherapy and increasing the incidence of adverse events, and surgical treatment may also suppress body immunity, activate and release some inflammatory factors, promote the growth and metastasis of residual tumors, and lead to further disease progression. Furthermore, there are many types of surgeries for patients with mGC due to different diseases, including translational surgery, palliative surgical resection, and the effect of different types of surgery is various, and the prognosis is also different [41]. The prognosis of patients with different surgical outcomes also varies, but the impact of these factors on prognosis cannot be further clarified due to the limited data from the database. Although the results of this study suggest that patients undergoing surgery have a lower risk of cancer-specific death, it does not show that all patients benefit from surgical treatment, and the effect of different surgical types on patient mortality in clinical observation needs to be further studied, so more prospective studies are needed in the future to study the effects of surgery and different surgical types on cancer-specific mortality in patients with mGC.
Although some studies report prognostic factors or models for mGC, few nomograms have been developed for the eighth edition of the AJCC TNM staging system, and the applicability of various biomarkers and inflammatory states in clinical practice remains unclear. On the contrary, the clinical variables included in our nomograms can be measured relatively easily and economically, and as a result, our nomograms are a more economical and practical option.
There are still some limitations to this study. First, this study is a retrospective study of two centers, not a prospective study, and the sample size is relatively small, so there is some selectivity bias. Second, our nomogram only included five clinicopathological factors, and did not take into account the general physical condition, underlying diseases, inflammatory indicators, post-chemotherapy reactions, and surgical complications of the patients, and the specific regimens of radiotherapy were not involved, and the impact of these factors on metastatic gastric cancer needs to be clarified in the future. Third, because the modeling cohort and the external validation cohort use data from different regions of China, geographical differences, ethnic composition and eating habits may have affected the results. Fourth, the median follow-up time differed between cohorts, which could lead to inaccuracies in cancer-specific deaths. Finally, all patients included in the external validation cohort were from a single institution in China with a small sample size, and larger, multicenter prospective randomized controlled trials are needed to validate the validity of nomograms.
Conclusions
In our study, we identified a series of factors as independent risk factors associated with cancer-specific survival, including differentiation grade, T stage, N stage, without chemotherapy and without surgery. We also identified the impact of chemotherapy regimens and cycles on the prognosis of mGC. Therefore, we developed and validated a nomogram-based prediction suitable for the Chinese mGC population. It helps clinicians to more accurately assess the survival status of patients and further promotes personalized treatment, which has important clinical utility.
Availability of data and materials
The data presented in this study are available on reasonable request from the corresponding author, Dr. Jun Ren.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Zheng RS, Zhang SW, Sun KX, Chen R, Wang SM, Li L, et al. Cancer statistics in China, 2016. Zhonghua Zhong Liu Za Zhi. 2023;45(3):212–20.
Zhao L, Li J, Bai C, Nie Y, Lin. Multi-modality treatment for patients with metastatic gastric cancer: a real-world study in China. Front Oncol. 2019;9:1155.
Yamaguchi K, Yoshida K, Tanahashi T, Takahashi T, Matsuhashi N, Tanaka Y, et al. The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer. 2017;21(2):315–23.
Sitarz R, Skierucha M, Mielko J, Offerhaus J, Maciejewski R, Polkowski W. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–48.
Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. 2021;41(8):747–95.
A. Japanese Gastric Cancer. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19.
Smyth EC, Nilsson M, Grabsch HI, van Grieken NCT, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635–48.
Bang Y-J, Kim Y-W, Yang H-K, Chung HC, Park Y-K, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–21.
Degiuli M, Sasako M, Ponti A, Vendrame A, Tomatis M, Mazza C, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg. 2014;101(2):23–31.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4.
Giardiello D, Steyerberg EW, Hauptmann M, Adank MA, Jager A, Oldenburg HOA, et al. Abstract P5-07-12: prediction and clinical utility of a contralateral breast cancer risk model. Can Res. 2020;80(4 Supplement):P5-07–12.
Zeng Y, Mayne N, Yang CJ, D’Amico TA, Ng CSH, Liu CC, et al. A nomogram for predicting cancer-specific survival of TNM 8th edition stage I non-small-cell lung cancer. Ann Surg Oncol. 2019;26(7):2053–62.
Wang J, Yang B, Li Z, Qu J, Liu J, Song N, et al. Nomogram-based prediction of survival in unresectable or metastatic gastric cancer patients with good performance status who received first-line chemotherapy. Ann Transl Med. 2020;8(6):311–311.
Ma T, Wu Z, Zhang X, Xu H, Feng Y, Zhang C, et al. Development and validation of a prognostic scoring model for mortality risk stratification in patients with recurrent or metastatic gastric carcinoma. BMC Cancer. 2021;21(1):1326.
Yang Y, Chen ZJ, Yan S. The incidence, risk factors and predictive nomograms for early death among patients with stage IV gastric cancer: a population-based study. J Gastrointest Oncol. 2020;11(5):964–82.
Shi L, Wang Z, Wang L, Jia Y, Li J, Qin Y. A prognostic nomogram and heat map to predict survival in stage II/III gastric cancer patients after curative gastrectomy followed by adjuvant chemotherapy. Cancer Manag Res. 2022;14:287–301.
Bando E, Ji X, Kattan MW, Seo HS, Song KY, Park CH, et al. Development and validation of a pretreatment nomogram to predict overall survival in gastric cancer. Cancer Med. 2020;9(16):5708–18.
Narita Y, Kadowaki S, Oze I, Kito Y, Kawakami T, Machida N, et al. Establishment and validation of prognostic nomograms in first-line metastatic gastric cancer patients. J Gastrointest Oncol. 2018;9(1):52–63.
Cats A, Jansen EPM, van Grieken NCT, Sikorska K, Lind P, Nordsmark M, et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):616–28.
Wagner AD, Syn NLX, Moehler M, Grothe W, Yong WP, Tai B-C, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8(8):CD004064.
Lee K-W, Zang DY, Ryu M-H, Han HS, Kim KH, Kim M-J, Koh SA, Lee SS, Koo D-H, Ko YH, Sohn BS, Kim JW, Park JH, Nam B-H, Choi IS. A phase 3 randomized clinical trial to compare efficacy and safety between combination therapy and monotherapy in elderly patients with advanced gastric cancer (KCSG ST13-10). Cancer Res Treat. 2023;55(4):1250–60.
Tang D, Liu GF, Li P, Wang S, Xu YX, Long AH, et al. S-1-based combination therapy vs S-1 monotherapy in advanced gastric cancer: a meta-analysis. World J Gastroenterol. 2014;20:310–8.
Kawakami H, Kimura Y, Tamura S, Fujitani K, Matsuyama J, Imamura H, et al. Effect of the number of cycles of docetaxel + S-1 therapy on long-term survival in adjuvant chemotherapy for stage III gastric cancer. A pooled analysis of the OGSG0604 and OGSG1002 trials. Gastric Cancer. 2023;26:788–97.
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9.
Chen J, Zhao G, Wang Y. Analysis of lymph node metastasis in early gastric cancer: a single institutional experience from China. World J Surg Oncol. 2020;18(1):57.
Kim YI, Lee JH, Kook MC, Lee JY, Kim CG, Ryu KW, et al. Lymph node metastasis risk according to the depth of invasion in early gastric cancers confined to the mucosal layer. Gastric Cancer. 2016;19(3):860–8.
Selcukbiricik F, Buyukunal E, Tural D, Ozguroglu M, Demirelli F, Serdengecti S. Clinicopathological features and outcomes of patients with gastric cancer: a single-center experience. World J Gastroenterol. 2013;19(14):2154–61.
Mahar AL, Zagorski B, Kagedan D, Dixon M, El-Sedfy A, Vasilevska-Ristovska J, et al. Evaluating TNM stage prognostic ability in a population-based cohort of gastric adenocarcinoma patients in a low-incidence country. Can J Public Health. 2018;109(4):480–8.
Hwang JE, Kim H, Shim HJ, Bae WK, Hwang EC, Jeong O, et al. Lymph-node ratio is an important clinical determinant for selecting the appropriate adjuvant chemotherapy regimen for curative D2-resected gastric cancer. J Cancer Res Clin Oncol. 2019;145(8):2157–66.
Chmelo J, Phillips AW. ASO author reflections: gastric cancer staging: more than just TNM? Ann Surg Oncol. 2020;27(9):3305–6.
Chang YR, Han DS, Kong SH, Lee HJ, Kim SH, Kim WH, et al. The value of palliative gastrectomy in gastric cancer with distant metastasis. Ann Surg Oncol. 2012;19(4):1231–9.
Fujitani K, Yang H-K, Mizusawa J, Kim Y-W, Terashima M, Han S-U, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17(3):309–18.
A. Japanese Gastric Cancer. Japanese gastric cancer treatment guidelines 2021 (6th edition). Gastric Cancer. 2023;26(1):1–25.
Hsu J-T, Liao J-A, Chuang H-C, Chen T-D, Chen T-H, Kuo C-J, et al. Palliative gastrectomy is beneficial in selected cases of metastatic gastric cancer. BMC Palliative Care. 2017;16(1):19
Kulig P, Sierzega M, Kowalczyk T, Kolodziejczyk P, Kulig J. Non-curative gastrectomy for metastatic gastric cancer: Rationale and long-term outcome in multicenter settings. Eur J Surg Oncol (EJSO). 2012;38(6):490–6.
Yazıcı O, Özdemir N, Duran AO, Menekşe S, Şendur MA, Karaca H, et al. The effect of the gastrectomy on survival in patients with metastatic gastric cancer: a study of ASMO. Future Oncol. 2016;12(3):343–54.
Kamarajah SK, Markar SR, Phillips AW, Salti GI, Dahdaleh F, Griffiths EA. Palliative gastrectomy for metastatic gastric adenocarcinoma: a national population-based cohort study. Surgery. 2021;170(6):1702–10.
Cowling J, Gorman B, Riaz A, Bundred JR, Kamarajah SK, Evans RPT, et al. Peri-operative outcomes and survival following palliative gastrectomy for gastric cancer: a systematic review and meta-analysis. J Gastrointest Cancer. 2020;52(1):41–56.
Fugazzola P, Ansaloni L, Sartelli M, Catena F, Cicuttin E, Leandro G, et al. Advanced gastric cancer: the value of surgery. Acta Biomed. 2018;89(8-S):110–6.
Funding
Social development project of Yangzhou, Yangzhou, China (YZ2023086); Social development project of Ili Kazak Autonomous Prefecture, Ili, China (YJC2023A33).
Author information
Authors and Affiliations
Contributions
ZZM, YZW and RJ: conceptualization, study design, study proposal, manuscript writing, and submission; DEX, JB, DP, SZ, HB and WRF: experimental investigation; RJ: study guidance and final review.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the committee of the Northern Jiangsu People's Hospital. Informed consent was obtained from the patient involved in the study.
Consent for publication
All authors gave their consent for publication. Consents for publication of the participants were not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Z., Dai, E., Jin, B. et al. A prognostic nomogram to predict the cancer-specific survival of patients with initially diagnosed metastatic gastric cancer: a validation study in a Chinese cohort. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03576-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03576-4