Abstract
Introduction
ECLIM-SEHOP platform was created in 2017. Its main objective is to establish the infrastructure to allow Spanish participation into international academic collaborative clinical trials, observational studies, and registries in pediatric oncology. The aim of this manuscript is to describe the activity conducted by ECLIM-SEHOP since its creation.
Methods
The platform’s database was queried to provide an overview of the studies integrally and partially supported by the organization. Data on trial recruitment and set-up/conduct metrics since its creation until November 2023 were extracted.
Results
ECLIM-SEHOP has supported 47 studies: 29 clinical trials and 18 observational studies/registries that have recruited a total of 5250 patients. Integral support has been given to 25 studies: 16 trials recruiting 584 patients and nine observational studies/registries recruiting 278 patients. The trials include front-line studies for leukemia, lymphoma, brain and solid extracranial tumors, and other key transversal topics such as off-label use of targeted therapies and survivorship. The mean time from regulatory authority submission to first patient recruited was 12.2 months and from first international site open to first Spanish site open was 31.3 months.
Discussion
ECLIM-SEHOP platform has remarkably improved the availability and accessibility of international academic clinical trials and has facilitated the centralization of resources in childhood cancer treatment. Despite the progressive improvement on clinical trial set-up metrics, timings should still be improved. The program has contributed to leveling survival rates in Spain with those of other European countries that presented major differences in the past.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pediatric cancer in Spain
Childhood cancers are considered rare diseases according to the RARECARE European project (incidence < 6 cases per 100,000) [1], but still represents the leading cause of disease-related death in children. In Spain, with a population of 6.6 million children under the age of 14, approximately 1100 new cases of childhood cancer are diagnosed yearly with an incidence rate of 165 cases per million according to the national registry [2]. Further 141 patients per year of cancer in adolescents (15–19 years of age) are also registered [2].
Nonetheless, survival has considerably improved within the last decades and current overall survival in Spain is 82% at 5 years [3, 4]. This figure has increased by 2% in the last 4 years and is gradually equalizing with the survival of other European countries (Germany 82.6%, Austria 85.3%, Belgium 83.8%) (EUROCARE-6 data) [5]. Despite this improvement, there has been limited progress in patient survival for difficult-to-treat pediatric malignancies such as metastatic sarcomas or certain brain tumors such as diffuse midline or pontine gliomas. In addition, childhood cancer survivors still face an unacceptable burden of long-term toxicities and sequelae to conventional therapies that require improvements in risk-adapted strategies and the incorporation of new agents [6].
The aim of SEHOP (Spanish Society of Pediatric Hematology and Oncology) (http://www.sehop.org) [7] is to ensure that every child, adolescent, and young adult is assisted in institutions that have the necessary means and sufficiently qualified health professionals using the same therapeutic schemes that are internationally recognized. In 1979, the Registro Español de Tumores Infantiles (RETI-SEHOP tumor registry) was created as a tool to improve knowledge in incidence and survival in Spain and to date more than 35,000 children with cancer have been registered. According to this registry, there are 46 centers treating childhood cancer in Spain, and only 12 of them treat more than 30 patients per year, the standard defined by SIOPE (European Society for Paediatric Oncology). The geographical dispersion, together with much needed improvement in survival makes the realization of academic clinical trials challenging, as they bring new standards and improve the quality of care through central review of imaging and pathology, and improve treatment compliance through enhanced trial monitoring activities.
Current status of international pediatric clinical trials in Spain
Notable, improvement has been made in the last years 7 years favoring the access to innovative agents for children and adolescents with cancer and the implementation of academic trials. In 2007, European legislation favored the development of new anti-cancer drugs in children through the pediatric investigation plan (PIP) requirement for pharmaceutical companies [8]. In addition, in 2015, the clinical trial regulations were updated (Royal Decree 1090/2015 of 4 December) [9], which eases the administrative burden and reduces costs for opening new academic studies. These two changes have favored greater access to new cancer therapies in childhood.
Other factors that have contributed to the generalization of academic trials are the active engagement of SEHOP with the adult cancer communities such as the Spanish sarcoma investigation group (GEIS) [10], the active participation within SIOP-Europe working groups and the incorporation of Spanish centers in the ITCC consortium (Innovative Therapies for Children with Cancer) [11, 12]. Finally, the key factor that has promoted the development of academic clinical trials is the implementation of a national pediatric program to facilitate the incorporation of clinical trials in the country: ECLIM (Multicenter international clinical trials).
Role of the ECLIM-SEHOP platform
ECLIM-SEHOP program was created in 2017 and fully established in 2018 [13, 14] by members of SEHOP. The objectives of the platform are:
-
1.
To establish the infrastructure to allow the participation of Spain into international academic collaborative clinical trials.
-
2.
To increase the number of academic international clinical trials open in the country.
-
3.
To improve the set-up (timelines) and conduct quality control of academic and international clinical trials open in Spain.
-
4.
To undertake trial-related activities as required from the international sponsors and national investigators: Regulatory submissions to competent authority and ethics committees, site contracting, monitoring, pharmacovigilance, management of biological samples, and central pathology and radiology review.
To have the capacity and expertise to perform all trial-related tasks required by international sponsors, an agreement with a clinical research organization (CRO) with a solid experience in academic clinical trials was sought and sustained over the years (http://www.sofpromed.com) [15]. Figure 1 shows the hierarchically structured organization of ECLIM-SEHOP supported by four main pillars.
The purpose of this manuscript is to describe the activity conducted by ECLIM-SEHOP focusing on data related to the studies portfolio, infrastructure evolution, recruitment and trial metrics.
Materials and methods
We provide information on clinical trials, observational studies and registries fully or partially supported by ECLIM-SEHOP from its creation in the beginning of 2017 to the 30 November 2023 including recruitment data. Integral support was defined as all trial-related activities being conducted by the platform. Descriptive metrics of all the activities conducted by the platform including timings regarding the opening, regulatory processes, and set-up procedures of the protocols were revised until 31 May 2023. The organization’s database with support from the CRO was required for this purpose. Descriptive statistics were used.
The timings measured covered the submission to the ethics committee (CEIm), the submission to the Spanish competent authorities (AEMPS), the time from the opening of the first site to the first patient recruited, and the timing from the first international site open to the first Spanish site open.
Results
Clinical studies portfolio
ECLIM-SEHOP has provided support to 47 studies from January 2017 to November 2023: 29 clinical trials and 18 observational studies or registries. Integral support has been given to 25 studies (16 clinical trials and 9 observational studies/registries). Partial support has been given to the other 22 studies (13 clinical trials and 9 observational studies/registries).
A relevant contribution has been the creation of the SEHOP-CLOUD imaging platform. This system allows anonymized DICOM medical image uploading and downloading. The web program contributes to the collection of medical images and facilitates central review of imaging and remote consultations. This infrastructure has been regularly used to facilitate centralized revision by expert radiologists in a particular area. It has also been useful to share images between centers to discuss in pediatric tumor boards.
Table 1 shows all the studies that receive integral support from ECLIM-SEHOP and Table 2 shows the studies partially supported. Figure 2 shows the distribution of the number of studies by type of malignancy.
Time and recruitment metrics
Sixteen international clinical trials received integral support from ECLIM-SEHOP. In these studies, SEHOP is acting as a national co-sponsor and the principal investigator is serving as the national coordinator. Out of these, eight have active recruitment, one has been closed (EURONET PHL-C2), and seven trials are currently under set-up process. In these studies, a total of 584 patients have been recruited, out of which 54 patients are newly enrolled in the last 6 months from the observation period for this analysis.
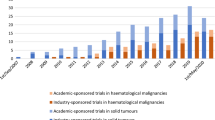
ECLIM-SEHOP supervises integrally nine observational studies/registries with comprehensive management. These studies have collected data on a total of 278 patients, including 55 newly enrolled patients in the last 6 months (data excluding imaging platform). Cumulative patient recruitment is shown in Fig. 3. Among these studies, seven are currently in recruitment phase, while two are undergoing administrative processes. Until now, 392 images have been undertaken on the SEHOP-CLOUD imaging platform, including 25 new cases in the last 6 months from the observation period for this analysis.
To measure the timings of the opening procedures, the available data from the nine integrally supported clinical trials containing recruitment was required from the CRO. Updated metrics for these timelines of clinical initiation are shown in Table 3.
Including the 25 studies (clinical trials, observational studies and patient registries) that receive integral support and the 22 studies that have partial support, a total of 5240 patients have been recruited under ECLIM-SEHOP in international studies.
Discussion
The creation and development of ECLIM-SEHOP platform has been a key milestone in promoting the treatment of childhood cancer by means of academic clinical trials in Spain. The studies included have increased exponentially and cover all pediatric malignancies and transversal topics.
The implementation of international trials has strengthened the Spanish pediatric oncology network, generated standard operational procedures and unified clinical, imaging and pathology criteria. Complying with the requirements of international sponsors, as many of the trials demand central imaging and pathology review, national hubs have been created for different cancers in various disciplines. As an example, central imaging review using SEHOP-CLOUD imaging infrastructure is unifying radiological interpretation in the SIOP Ependymoma II trial and this platform is also widely used in Hodgkin and non-Hodkin lymphoma protocols and other solid malignancies. Some national tumor groups such as the brain or the soft tissue sarcoma group also discuss complex cases in videoconference based tumor committees sharing radiological images by means of this facility and pediatric radiologists of different centers are being included in the decision-making procedures.
Regarding pathology, many of the studies such as FAR-RMS, Euronet-C2, Umbrella, LOGGIC-CORE, and others require centralized pathology review and all the tumor samples are referred to a unique center trying to achieve an homogeneous interpretation and higher quality diagnostic criteria [16]. Samples in some studies are further exported to an international hub for advanced genomic studies to secure that every patient has access to all the biologic determinations that the protocols demand.
One of the current challenges is the set-up of the academic phase III, ALL-Together platform for the treatment of acute lymphoblastic leukemia (ALL). This study is an initiative where several study groups from European countries including NOPHO, UKALL, DCOG, COALL, BSPHO, SHOP, and SFCE, previously collected their experience of successful treatment of children and young adults with ALL and designed a collaborative protocol. This new platform study is both a comprehensive system for stratification and treatment of ALL as well as the basis for several randomized trials included in the study design [17]. Implementing this study is a challenge per se as it targets pediatric and young adult front-line acute lymphoblastic leukemia expecting a high recruitment rate and including several sub-studies.
Other important observational studies and registries focused on key transversal aspects have been integrally supported by ECLIM-SEHOP. The SACHA study (Secured access to innovative medicines for children, adolescents and young adults with cancer) has been opened recently in four centers (H. La Fe, H. Miguel Servet, H. Dr. Balmis de Alicante and H. 12 Octubre) and will be active in 15 in the following months. The aim of SACHA is to secure the access of children with pediatric malignancies, non-eligible to clinical trials, to innovative therapies with compassionate use or off-label administration of anti-cancer drugs, following the SACHA-France study [18]. Focused on other issue, the Survivorship Passport study is looking toward the implementation of the follow-up of late effects of childhood cancer survivors (SIOPE survivors passport project) [19].
Significant progress has been observed regarding the set-up study metrics. Compared to the previous review in 2019 [13], the median time from competent authorities’ approval to the first site opening has been reduced by 2 months over the last 4 years. This reduction is indicative of an increasing inclusion of the required information in submissions and in cases where it is lacking, issues can be promptly corrected due to the smooth communication with the authorities. Additionally, the time from first site opening to the first patient recruited has decreased to 1.8 months. This reflects greater efficiency in the recruitment process, likely attributable to the strong commitment of Spanish sites. All these improvements have positioned Spain around the European average in terms of trial initiation timings [20, 21] revealing a significant opportunity to strengthen the country's position in pediatric cancer clinical research.
One of the most important metrics that summarizes the main objectives of ECLIM-SEHOP is the median time from the first international site open to the first Spanish site open. This duration has been significantly reduced from 41.5 to 31.3 months [13], reflecting the improved performance in delivering clinical research. However, the lengthy process of setting up international trials still exists and further work must be done to reduce the opening times of the trials in Spain after the opening in the leading country. Additionally, the adaptations to implement the new Clinical Trial Regulation [22], including the CTIS (Clinical Trials Information System) database managed by the European Medicines Agency (EMA), have produced delays in the opening of some of the trials. Despite this, it will soon allow sponsors to apply for authorization of a new clinical trial in up to 30 countries at the same time, simplifying processes and enhancing the effectiveness of all clinical trials, particularly benefiting those carried out in the Member States.
Historically, Spain has not been able to participate in multiple international clinical trials because of lack of resources and infrastructure to adapt to modern regulatory standards and provide good quality data. For some other studies, the time to set up the trials has been so long due to administrative barriers that the trials were completed or nearly completed internationally by the time they were open in the country [13]. These issues have been reflected in 5-year survival rate figures, where Spain has lagged behind other northern and central European countries. However, comparing the current trends, Spain shows significant progress in survival data. Based on the EUROCARE-5 project (data from 1978 to 2007) [23], the 5-year survival rate in the country for all tumors excluding CNS malignancies was 3% inferior than the average of all European countries (79% vs 82%). However, according to the updated EUROCARE-6 data (data from 2000 to 2014), this gap between Spain and Europe has decreased to 0.1% (85.2% vs 85.3%) (Table 4). The global increase in European countries was 3.3%, while Spain has doubled this increase (6.2%). Considering the 2023 RETI-SEHOP report, survival in the country has reached 82% so presently it is possible that Spain is or will be soon above the European 5-year survival average [4]. We believe that the gradual incorporation of Spain into international clinical trials with the support of ECLIM-SEHOP has largely contributed in this improvement.
The main challenge of the platform is to ensure long-term sustainability. To enhance the capacity for innovation in the field of clinical research and strive toward the goal of potentially including every child and adolescent in a clinical trial, it is imperative for public institutions to secure increased funding and bolster their capabilities. This includes the development of more academic trials and the implementation of collaborative international plans. [13]. ECLIM-SEHOP has been supported with funds from competitive and non-competitive grants from philanthropic organizations and, therefore, with increasing complexity of clinical trials, is in constant need of identifying financial resources to make it sustainable [13].
Conclusion
ECLIM-SEHOP has proven to be an essential tool, signifying a paradigm shift in pediatric oncology research within our country. Through this organization, standard treatments in Spain are transitioning toward European state-of-the-art diagnostic and therapeutic strategies. The platform supports many front-line treatments for children with cancer, playing a pivotal role in enhancing survival rates in pediatric cancer and aligning Spain's situation with that of other European countries.
Data availability
Not applicable.
References
Gatta G, Van der Zwan JM, Casali PG, Siesling S, Deitos AP, Kunkler I, et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. 2011;47:2493–511. https://doi.org/10.1016/j.ejca.2011.08.008.
Cañete Nieto A, Pardo Romaguera E, Muñoz López A, Valero Poveda S, Porta Cebolla S, Barreda Reines MS, et al. Cáncer infantil en España. Estadísticas 1980–2021. Registro Español de Tumores Infantiles (RETI-SEHOP). Valencia: Universitat de València; 2022.
Peris-Bonet R. Incidencia y supervivencia del cáncer infantil. In: Madero L, Lassaleta A, Sevilla J, editors. Hematol y Oncol Pediátricas. 3rd ed. Madrid: Ergon; 2015. p. 263–70.
Cañete Nieto A, Pardo Romaguera E, Alfonso Comos P, Valero Poveda S, Fernández Férriz A, Porta Cebolla S, et al. Cáncer infantil en España. Estadísticas 1980–2022. Registro Español de Tumores Infantiles (RETI-SEHOP). Valencia: Universitat de València (Edición preliminar); 2023.
Botta L, Gatta G, Capocaccia R, Stiller C, Cañete A, Dal Maso L, et al. Long-term survival and cure fraction estimates for childhood cancer in Europe (EUROCARE-6): results from a population-based study. Lancet Oncol. 2022;23(12):1525–36. https://doi.org/10.1016/S1470-2045(22)00637-4.
Armstrong GT, Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374:833–42.
Sociedad Española de Hematología y Oncología Pediátricas (SEHOP). http://www.sehop.org. Accessed 29 December 2023.
Nordenmalm S, Tomasi P, Pallidis C. More medicines for children: impact of the EU paediatric regulation. Arch Dis Child. 2018;103:557–64.
Real Decreto 1090/2015, de 4 de diciembre, por el que se regulan los ensayos clínicos con medicamentos, los Comités de Ética de la Investigación con medicamentos y el Registro Español de Estudios Clínicos. https://www.boe.es/buscar/doc.php?id=BOE-A-2015-14082-A-2015-14082. 2015. Accessed 29 Dec 2023.
Grupo Español de Investigación en Sarcomas (GEIS). http://www.grupogeis.org. Accessed 28 December 2023.
Zwaan CM, Kearns P, Caron H, Verschuur A, Riccardi R, Boos J, et al. The role of the “innovative therapies for children with cancer” (ITCC) European consortium. Cancer Treat Rev. 2010;36:328–34.
Innovative Therapies for Children with Cancer (ITCC). https://www.itcc-consortium.org/about-itcc.php. Accessed 20 December 2023.
Bautista F, Cañete A, Ramírez-Villar GL, et al. Sociedad Española de Hematología y Oncología Pediátrica (SEHOP). ECLIM-SEHOP, a new platform to set up and develop international academic clinical trials for childhood cancer and blood disorders in Spain. Clin Transl Oncol. 2019;21(12):1763–70. https://doi.org/10.1007/s12094-019-02221-9. (Epub 2019 Oct 9. PMID: 31598904).
Rubio-San-Simón A, Hladun Alvaro R, Juan Ribelles A, et al. The paediatric cancer clinical research landscape in Spain: a 13-year multicentre experience of the new agents group of the Spanish Society of Paediatric Haematology and Oncology (SEHOP). Clin Transl Oncol. 2021;23(12):2489–96. https://doi.org/10.1007/s12094-021-02649-y. (Epub 2021 Jun 2 PMID: 34076861).
Sofpromed. http://www.sofpromed.com. Accessed 20 December 2023.
Hardin EC, Schmid S, Sommerkamp A, et al. LOGGIC core bioclinical data bank: added clinical value of RNA-Seq in an international molecular diagnostic registry for pediatric low-grade glioma patients. Neuro Oncol. 2023;25(11):2087–97. https://doi.org/10.1093/neuonc/noad078. (PMID: 37075810; PMCID: PMC10628936).
ALLTogether1—a treatment study protocol of the alltogether consortium for children and young adults (0–45 years of age) with newly diagnosed acute lymphoblastic leukaemia (ALL). ClinicalTrials.gov Identifier: NCT04307576. https://classic.clinicaltrials.gov/ct2/show/NCT04307576
Berlanga P, Ndounga-Diakou LA, Aerts I, et al. Measuring safety and outcomes for the use of compassionate and off-label therapies for children, adolescents, and young adults with cancer in the SACHA-France Study. JAMA Netw Open. 2023;6(7): e2321568. https://doi.org/10.1001/jamanetworkopen.2023.21568. (PMID: 37399010).
Survivorship passport. http://www.survivorshippassport.org. Accessed 15 December 2023.
PROYECTO BEST Investigación Clínica en Medicamentos. BDMetrics Datos y Análisis 33ª Publicación (30 de Junio de 2023). https://www.medicamentos-innovadores.org/sites/medicamentosinnovadores/InvestigacionClinicaProyectoBESTHome.html.
https://healthmedia.blog.gov.uk/2023/11/22/what-were-doing-to-speed-up-clinical-trials-in-the-uk/. Accessed 15 Dec 2023.
Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC. 2014.
Gatta G, Botta L, Rossi S, Aareleid T, Bielska-Lasota M, Clavel J, et al. Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5—a population-based study. Lancet Oncol. 2014;15:35–47.
Acknowledgements
We would like to thank all children, parents, and legal tutors that participate in clinical trials. We thank the members of the technical secretariat of SEHOP, Fundación SEHOP and RETI-SEHOP, particularly Elena Pardo and Inmaculada Toledano, for their continued work, support and enthusiasm. We thank Patricio Ledesma, Head of Clinical Operations and all the staff at Sofpromed Investigación Clínica, for the close and fruitful collaboration in building up ECLIM-SEHOP. We are grateful to our colleagues of SEHOP and their teams (physicians, nurses, study coordinators, etc.) for their interest and commitment on clinical research to improve care for children with cancer and hematological diseases.
Funding
ECLIM-SEHOP was supported by the grant “Cáncer infantil y cánceres poco frecuentes 2018” from Asociación Española Contra el Cáncer (AECC) (Grant CICPF18016FERN 2018). Support is also received from Federación Española de Padres de Niños con Cáncer, Asociación Pablo Ugarte, Fundación Inocente, ACAYE, Uno entre cien mil and Fundación MAR.
Author information
Authors and Affiliations
Consortia
Contributions
A. Juan-Ribelles: Validation, Writing original draft, data curation, visualization, investigation, project administration. A. Rubio-San Simón, A. Alonso-Saladrigues: Writing—review, project administration, supervision. R. Hladun, S. Rives, J. L. Dapena, J. M. Fernández, Á. Lassaletta, O. Cruz, G. Ramírez-Villar, J. L. Fuster, C. Diaz-de-Heredia, M. García-Ariza, E. Quiroga, M. del M. Andrés, J. Verdú-Amorós, A. Molinés, B. Herrero, M. López, C. Márquez: Writing—Review, investigation. M. Toboso: Formal analysis, data curation. F. Lendínez, J. Gómez Sirvent, M. Tallón: Writing—Review, project administration. G. Rodríguez: Data curation. T. Acha: Conceptualization. F. Bautista, A. Cañete, L. Moreno, A. Fernández-Teijeiro: Conceptualization, supervision, Writing—Review, project administration, investigation, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
A. Juan Ribelles has had a consulting/advisory role for Alexion and Bayer, has participated in educational activities organized by Eusa Pharma, Abbott, and Alexion, and has received travel expenses by Nestle and Alexion. F. Bautista has been a member of a data monitoring committee (DMC) in a clinical trial sponsored by Sanofi, had a consulting or advisory role for Bayer, Amgen, Roche Genentech, EusaPharma, and Eisai, and received honoraria from Servier for educational events (through his current affiliation). A. Cañete has had an advisory role for Norgine, Bayer, Roche, Eusa-Pharma, SERB, and participated in educational activities organized by Eusa Pharma and Norgine and received travel expenses from Eusa-pharma and Nestlé. A. Rubio-San Simon has had a advisory role for Eusa Pharma and Sanofi and has received travel expenses by Roche and Eusa Pharma. A. Alonso-Saladrigues (AAS): advisory boards (Novartis). S. Rives reports personal fees, honoraria from Novartis, Servier, Celgene/ Bristol Myers Squibb, Kite/ Gilead, and Amgen. Also reports being part of DMSB in clinical trial sponsored by Novartis and of a DMC in a clinical trial sponsored by Autolus. A. Lassaletta has had an advisory role for Jazz, Servier, and Alexion; has received travel expenses by Servier, and has participated in educational activities organized by Alexion. G. Ramírez-Villar has had an advisory role for Alexion, has received travel expenses by Nestlé and has participated in educational activities organized by Alexion. J.L.Fuster was a consultant/advisory member for Amgen, Jazz Pharmaceuticals, and Novartis, received honoraria from Amgen, Servier, Jazz Pharmaceuticals, Pfizer, and Novartis for speaking at symposia and support from Servier and Jazz Pharmaceuticals for attending symposia. C. Diaz-de-Heredia is a member of study steering committees for clinical trials sponsored by Novartis, had a consulting or advisory role for Novartis, Jazz Pharmaceuticals, Biotest and Vertex, has participated in educational activities organized by Novartis and Jazz Pharmaceuticals and received travel expenses from Jazz Pharmaceuticals and Novartis. J. Verdú-Amorós has received honoraria by Servier and received travel expenses by Servier and EusaPharma. A. Molinés has participated in educational activities organized by Servier receiving travel and accommodation expenses. L. Moreno has been member of data monitoring committees for clinical trials sponsored by Novartis, Actuate Therapeutics, Shionogi and Incyte; had a consulting role for Novartis, Norgine, Boehringer, Ymabs, Bayer, Abbvie, BMS and Shionogi; participated in educational activities organized by Bayer and Eusa Pharma and received travel expenses from Eusa Pharma. He is President Elect of SIOPEN (European neuroblastoma research cooperative group), an organization which receives royalties for the sales of dinutuximab beta. A. Fernández-Teijeiro has had an advisory role for AstraZeneca, SOBI, Takeda, Norgine, Clinigen, Bayer, Roche, MERCK SHARP & DOHME, Novartis and Takeda; participated in educational activities organized by Amgen, Eusa Pharma, Servier and Takeda and received travel expenses from Servier, Amgen and Gilead. The other authors declare no conflict of interest.
Ethical standards
All human studies had been approved by the appropriate ethics committee and had, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Parents and/or legal guardians of children and adult patients participating in these clinical trials gave their informed consent prior to their inclusion in the studies reported in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Juan-Ribelles, A., Bautista, F., Cañete, A. et al. ECLIM-SEHOP: how to develop a platform to conduct academic trials for childhood cancer. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03445-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03445-0