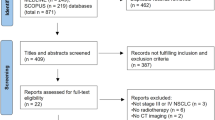
Abstract
Objectives
The S-REAL study aimed to assess the effectiveness of durvalumab as consolidation therapy after definitive chemoradiotherapy (CRT) in a real-world cohort of patients with locally advanced, unresectable stage III non-small cell lung cancer (LA-NSCLC) included in a Spanish early access program (EAP).
Methods
In this multicentre, observational, retrospective study we analysed data from patients treated in 39 Spanish hospitals, who started intravenous durvalumab (10 mg/kg every 2 weeks) between September 2017 and December 2018. The primary endpoint was progression-free survival (PFS). Secondary endpoints included patient characterization and adverse events of special interest (AESI).
Results
A total of 244 patients were followed up for a median of 21.9 months [range 1.2–34.7]. Median duration of durvalumab was 45.5 weeks (11.4 months) [0–145]. Median PFS was 16.7 months (95% CI 12.2–25). No remarkable differences in PFS were observed between patients with programmed cell death-ligand 1 (PD-L1) expression ≥ 1% or < 1% (16.7 versus 15.6 months, respectively). However, PFS was higher in patients who had received prior concurrent CRT (cCRT) versus sequential CRT (sCRT) (20.6 versus 9.4 months). AESIs leading to durvalumab discontinuation were registered in 11.1% of patients.
Conclusions
These results are in line with prior published evidence and confirm the benefits of durvalumab in the treatment of LA-NSCLC patients in a real-world setting. We also observed a lower incidence of important treatment-associated toxicities, such as pneumonitis, compared with the pivotal phase III PACIFIC clinical study.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.
Abbreviations
- AE:
-
Adverse events
- AESI:
-
Adverse events of special interest
- cCRT:
-
Concurrent chemoradiotherapy
- CT:
-
Chemotherapy
- EAP:
-
Expanded access program
- IQR:
-
Interquartile range
- LA-NSCLC:
-
Locally advanced non-small cell lung cancer
- OS:
-
Overall survival
- PD-L1:
-
Programmed cell death ligand-1
- PFS:
-
Progression-free survival
- Q1:
-
Quartile 1 (25%)
- Q3:
-
Quartile 3 (75%)
- sCRT:
-
Sequential chemoradiotherapy
- SD:
-
Standard deviation
- SOC:
-
Standard of care
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Estimates for cancer incidence and mortality in 2020, (Lung cancer incidence and mortality), in all countries. Available at https://ecis.jrc.ec.europa.eu/. Accessed 25 Nov 2022.
Agbary A, Shalata W, Addeo A, Charpidou A, Cuppens K, Brustugun OT, et al. Real-world journey of unresectable stage III NSCLC patients: current dilemmas for disease staging and treatment. J Clin Med. 2022. https://doi.org/10.3390/jcm11061738.
Yoon SM, Shaikh T, Hallman M. Therapeutic management options for stage III non-small cell lung cancer. World J Clin Oncol. 2017;8(1):1–20.
Curran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103(19):1452–60.
Bradley JD, Hu C, Komaki RR, Masters GA, Blumenschein GR, Schild SE, et al. Long-term results of NRG oncology RTOG 0617: standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol. 2020;38(7):706–14.
Hansen RN, Zhang Y, Seal B, Ryan K, Yong C, Darilay A, et al. Long-term survival trends in patients with unresectable stage III non-small cell lung cancer receiving chemotherapy and radiation therapy: a SEER cancer registry analysis. BMC Cancer. 2020;20(1):276.
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–29.
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–50.
Hui R, Özgüroğlu M, Villegas A, Daniel D, Vicente D, Murakami S, et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(12):1670–80.
European Agency Medicines: Imfizi: EPAR – Product information, 2018. Available at https://www.ema.europa.eu/en/medicines/human/EPAR/imfinzi.
Remon J, Soria JC, Peters S, ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer: an update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol. 2021;32(12):1637–42.
Daly ME, Singh N, Ismaila N, Antonoff MB, Arenberg DA, Bradley J, et al. Management of stage III non-small-cell lung cancer: ASCO Guideline. J Clin Oncol. 2022;40(12):1356–84.
Faivre-Finn C, Vicente D, Kurata T, Planchard D, Paz-Ares L, Vansteenkiste JF, et al. Four-Year Survival With Durvalumab after chemoradiotherapy in stage III NSCLC-an update from the PACIFIC Trial. J Thorac Oncol. 2021;16(5):860–7.
Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC-update from PACIFIC. J Thorac Oncol. 2020;15(2):288–93.
Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-year survival outcomes from the PACIFIC Trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40(12):1301–11.
Huber RM, De Ruysscher D, Hoffmann H, Reu S, Tufman A. Interdisciplinary multimodality management of stage III nonsmall cell lung cancer. Eur Respir Rev. 2019;28(152): 190024.
Ryan KJ, Skinner KE, Fernandes AW, Punekar RS, Pavilack M, Walker MS, et al. Real-world outcomes in patients with unresected stage III non-small cell lung cancer. Med Oncol. 2019;36(3):24.
Jazieh AR, Onal HC, Tan DSW, Soo RA, Prabhash K, Kumar A, et al. Real-World treatment patterns and clinical outcomes in patients with stage III NSCLC: results of KINDLE, a Multicountry Observational Study. J Thorac Oncol. 2021;16(10):1733–44.
Passaro A, Spitaleri G, Gyawali B, de Marinis F. Immunotherapy in non-small-cell lung cancer patients with performance status 2: clinical decision making with scant evidence. J Clin Oncol. 2019;37(22):1863–7.
Girard N, Bar J, Garrido P, Garassino MC, McDonald F, Mornex F, et al. Treatment characteristics and real-world progression-free survival in patients with unresectable stage III NSCLC who received durvalumab after chemoradiotherapy: findings from the PACIFIC-R Study. J Thorac Oncol. 2023;18(2):181–93.
Faehling M, Schumann C, Christopoulos P, Hoffknecht P, Alt J, Horn M, et al. Durvalumab after definitive chemoradiotherapy in locally advanced unresectable non-small cell lung cancer (NSCLC): real-world data on survival and safety from the German expanded-access program (EAP). Lung Cancer. 2020;150:114–22.
Taugner J, Käsmann L, Eze C, Rühle A, Tufman A, Reinmuth N, et al. Real-world prospective analysis of treatment patterns in durvalumab maintenance after chemoradiotherapy in unresectable, locally advanced NSCLC patients. Invest New Drugs. 2021;39(4):1189–96.
Bruni A, Scotti V, Borghetti P, Vagge S, Cozzi S, D’Angelo E, et al. A real-world, multicenter, observational retrospective study of durvalumab after concomitant or sequential chemoradiation for unresectable stage III non-small cell lung cancer. Front Oncol. 2021;11: 744956.
Abe T, Saito S, Iino M, Aoshika T, Ryuno Y, Ohta T, et al. Effect of durvalumab on local control after concurrent chemoradiotherapy for locally advanced non-small cell lung cancer in comparison with chemoradiotherapy alone. Thorac Cancer. 2021;12(2):245–50.
Huang Y, Zhao JJ, Soon YY, Wong A, Aminkeng F, Ang Y, et al. Real-world experience of consolidation durvalumab after concurrent chemoradiotherapy in stage III non-small cell lung cancer. Thorac Cancer. 2022;13(22):3152–61.
Sankar K, Bryant AK, Strohbehn GW, Zhao L, Elliott D, Moghanaki D, et al. Real world outcomes versus clinical trial results of durvalumab maintenance in veterans with stage III non-small cell lung cancer. Cancers (Basel). 2022;14(3):614.
Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv1–21.
Faivre-Finn C, Spigel DR, Senan S, Langer CJ, Raben D, Perez B, et al. Efficacy and safety evaluation based on time from completion of radiotherapy to randomization with durvalumab or placebo in pts from PACIFIC. Ann Oncol. 2018;29:viii488.
Garassino MC, Mazieres J, Reck M, Chouaid C, Bischoff H, Reinmuth N, et al. Durvalumab after sequential chemoradiotherapy in stage III, unresectable NSCLC: the phase 2 PACIFIC-6 trial. J Thorac Oncol. 2022;17(12):1415–27.
Wu YL, Wang L, Sendur MAN, Kim Y-C, Zhu Z, Chenget Y, et al. PACIFIC-5: Phase III study of durvalumab after either concurrent or sequential chemoradiotherapy (CRT) in patients with stage III NSCLC. Ann Oncol. 2019;30:ix13–114.
Acknowledgements
This work was sponsored and conducted through the Spanish Lung Cancer Group (GECP). The authors would like to thank all the patients and families involved in the S-REAL study as well as all participating researchers and teams. Without their help, this study would not have been possible. The authors would also like to thank Susana Cañón Sánchez, PhD (Medical Statistics Consulting S.L., Valencia, Spain) for providing scientific and medical writing support, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Author information
Authors and Affiliations
Consortia
Contributions
Study conception and design: PG. All authors participated in data collection, data review and interpretation. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
AGR has received speaker and consultant honoraria from AstraZeneca, Amgen and Takeda, and travel support from AstraZeneca, Takeda and MSD; AT has received personal fees and non-financial support from Pfizer, Roche, BMS, MSD, and AstraZeneca; RAA has received consultant and speaker honoraria from Boehringer, Novartis, Pharmamar and Roche, conference registration support from MSD Oncology, and has participated as PI coordinator in projects sponsored by Boehringer, Cebiotex, Janssen Oncology, Novartis, Rain Therapeutics and Roche; RBC has received speaker and/or consultant honoraria from Pfizer, Janssen, Sanofi, AstraZeneca, Bristol Myers, Roche, Takeda and Jannsen, and research funding from Roche; LV has received consultant honorarium from Boehringer Ingelheim, AstraZeneca and Sanofi and speaker honoraria from AstraZeneca, Sanofi, MSD, Pfizer and Roche. MASG has received speaker and lecturer honoraria from Takeda and Roche, and travel/meeting attendance support from Roche and PharmaMar; MD has received grants from Roche and travel/meeting attendance support from Lilly; SF has received speaker honorarium from Pfizer and Sanofi, and travel support from AstraZeneca and MSD; CA has received consultant honorarium from AstraZeneca, BMS, MSD and Novartis, research funding from MSD, Mirati and AstraZeneca, and travel support from Roche, Novartis, Takeda and Pierre Fabre; AB has received speaker and consultant honoraria from BMS, Roche, MSD, Sanofi and Takeda, and travel support from MSD, Roche and BMS; DI has received speaker/consultant honoraria from Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Hoffmann-La Roche, Janssen, Lilly, Merck, MSD, Novartis, Pfizer, Sanofi and Takeda, has participated in clinical trials sponsored by Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Hoffmann-La Roche, GSK, Janssen, Lilly, Merck, Mirati Therapeutics, MSD, Novartis, Pfizer and Sanofi, and has received research grants from AstraZeneca, BMS, Hoffmann-La Roche and GSK; LBH has received speaker honorarium from Roche, BMS and AstraZeneca, consultant honorarium from Boehringer and Sanofi, and meeting attendance support from MSD, Novartis, Sanofi and AstraZeneca; JLFP has received consultant and speaker honoraria from AstraZeneca; BM has received personal fees from Roche, BMS, MSD, Boehringer Ingelheim and Pfizer; XMR has received speaker and/or consultant honoraria from Novartis, Roche, Pfizer, Astra Zeneca and MSD, grants from BMS, travel/meeting attendance support from Roche, Lilly and MSD, and declares his membership of the boards of the Spanish Group for Transversal Oncology and Research in Orphan and Infrequent Tumors (GETTHI) and the Spanish Group for Cancer Immuno-Biotherapy (GETICA); MD has received consultant and/or speaker honoraria from AstraZeneca, Boehringer Ingelheim, Janssen Cilag, BMS MSD, Pfizer, Roche, Sanofi and Takeda; IGB has received consultant and/or speaker honoraria from MSD Oncology, Lilly, AstraZeneca and BMS/Celgene, Roche and Amgen, and travel support from MSD Oncology, Lilly and Daiichi Sankyo; DR-A has received consultant and speaker honoraria from Roche/Genentech, AstraZeneca, BMS, Boehringer Ingelheim, MSD, Merck Serono, Eli Lilly, Gilead, Sanofi, Regeneron, Incyte, Pfizer, Takeda and Novartis, and travel support from Roche, BMS, MSD, Sanofi, Regeneron and Novartis; GAJC has received speaker/consultant honoraria from AstraZeneca and Roche; LLM has received consultant/speaker honoraria from AstraZeneca; JMST has received speaker/consultant honoraria from AstraZeneca; PG has received consultant honorarium from Abbvie, Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Daiichi Sankyo, GlaxoSmithKline, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi and Takeda, and direct funding from Medscape and Touch Medical. All other authors have no conflict of interest to declare.
Ethical approval (Research involving human participants and/or animals) and Informed consent
This study followed the Helsinki Declaration, Good Clinical Practices and the Spanish Organic Law 3/2018 of 5 December on the Protection of Personal Data and digital rights guarantee. The study was approved by the Ethics Committee of Hospital Puerta de Hierro de Majadahonda (Madrid). Written informed consent was obtained from all enrolled patients. Patient medical information obtained for this study was confidential and was only disclosed to the researchers and third parties involved, as detailed in the informed consent form signed by the patients. Confidentiality standards were maintained by assigning each patient a unique subject identification number.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gómez Rueda, A., Taus, Á., Álvarez Álvarez, R. et al. The S-REAL study: Spanish real-world data on unresectable stage III NSCLC patients treated with durvalumab after chemoradiotherapy. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03404-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03404-9