Abstract
Background
Cancer is a risk factor for developing severe COVID19. Additionally, SARS-CoV2 has a special tropism for renal cells and complications like thrombosis or cytokine storm could be enhanced by standard treatments in kidney cancer (i.e., antiangiogenics or immunotherapy). Thus, understanding the impact of COVID19 in patients with this tumor is key for their correct management.
Methods
We designed a retrospective case–control study comparing the outcome of three groups of advanced kidney cancer patients on systemic treatment: cohort A (developed COVID19 while on antiangiogenics), cohort B (developed COVID19 while on immunotherapy) and cohort C (non-infected). Matching factors were age, gender, and treatment.
Results
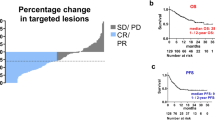
95 patients were recruited in 16 centers in Spain from September 2020 to May 2021. Finally, 85 were deemed as eligible (23 cohort A, 21 cohort B, 41 cohort C).
Patients with COVID required more dose interruptions (25 vs. six) and hospitalizations (10 vs. none) than those without COVID (both p = 0.001). No difference between cohorts A and B was observed regarding hospitalization or length of stay. No ICU admission was registered and one patient in cohort B died due to COVID19. Regarding cancer evolution, three patients in cohort A presented progressive disease after COVID19 compared to two in cohort B. One case in cohort B, initially deemed as stable disease, achieved a partial response after COVID19.
Conclusions
Kidney cancer patients who developed COVID19 while on systemic therapy required more treatment interruptions and hospitalizations than those non-infected. However, no significant impact on cancer outcome was observed. Also, no difference was seen between cases on antiangiogenics or immunotherapy.


Similar content being viewed by others
References
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. https://doi.org/10.1016/S0140-6736(20)30566-3.
Zhang H, Han H, He T, Labbe KE, Hernandez AV, Chen H, et al. Clinical characteristics and outcomes of COVID-19-infected cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2021;113(4):371–80. https://doi.org/10.1093/jnci/djaa168.
Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–9. https://doi.org/10.1001/jama.2020.6775.
Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–9. https://doi.org/10.1056/NEJMsr2005760.
Ahmadian E, Hosseiniyan Khatibi SM, Razi Soofiyani S, Abediazar S, Shoja MM, Ardalan M, et al. Covid-19 and kidney injury: pathophysiology and molecular mechanisms. Rev Med Virol. 2021;31(3):e2176. https://doi.org/10.1002/rmv.2176.
Choong OK, Jakobsson R, Bergdahl AG, Brunet S, Kärmander A, Waldenström J, et al. SARS-CoV-2 replicates and displays oncolytic properties in clear cell and papillary renal cell carcinoma. PLoS ONE. 2023;18(1):e0279578. https://doi.org/10.1371/journal.pone.0279578.
Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–98. https://doi.org/10.1007/s00134-020-06062-x.
Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–4. https://doi.org/10.1126/science.abb8925.
Labaki C, Peters S, Choueiri TK. Treatment decisions for patients with cancer during the COVID-19 pandemic. Cancer Discov. 2021;11(6):1330–5. https://doi.org/10.1158/2159-8290.CD-21-0210.
Rubinstein SM, Steinharter JA, Warner J, Rini BI, Peters S, Choueiri TK. The COVID-19 and Cancer Consortium: a collaborative effort to understand the effects of COVID-19 on patients with cancer. Cancer Cell. 2020;37(6):738–41. https://doi.org/10.1016/j.ccell.2020.04.018.
Ürün Y, Hussain SA, Bakouny Z, Castellano D, Kılıçkap S, Morgan G, et al. Survey of the impact of COVID-19 on oncologists’ decision making in cancer. JCO Glob Oncol. 2020;6:1248–57. https://doi.org/10.1200/GO.20.00300.
Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–9. https://doi.org/10.1200/JCO.2008.21.4809.
Grivas P, Khaki AR, Wise-Draper TM, French B, Hennessy C, Hsu CY, et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol. 2021;32(6):787–800. https://doi.org/10.1016/j.annonc.2021.02.024.
Challenor S, Tucker D. SARS-CoV-2-induced remission of Hodgkin lymphoma. Br J Haematol. 2021;192(3):415. https://doi.org/10.1111/bjh.17116.
Sollini M, Gelardi F, Carlo-Stella C, Chiti A. Complete remission of follicular lymphoma after SARS-CoV-2 infection: from the “flare phenomenon” to the “abscopal effect. J Nucl Med Mol Imaging. 2021;48:2652–4. https://doi.org/10.1007/s00259-021-05275-6.
Aeppli S, Eboulet EI, Eisen T, Escudier B, Fischer S, Larkin J, et al. Impact of COVID-19 pandemic on treatment patterns in metastatic clear cell renal cell carcinoma. ESMO Open. 2020;5:e000852.
Luo J, Rizvi H, Egger JV, Preeshagul IR, Wolchok JD, Hellmann MD. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;10(8):1121–8. https://doi.org/10.1158/2159-8290.CD-20-0596.
Rogiers A, Pires da Silva I, Tentori C, Tondini CA, Grimes JM, Trager MH, et al. Clinical impact of COVID-19 on patients with cancer treated with immune checkpoint inhibition. J Immunother Cancer. 2021;9(1):e001931.
Funding
This study has been funded through an independent educational grant by Pfizer Inc. The sponsor played no role in the execution of the study or manuscript writing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declared no conflict of interest regarding this article.
Ethical approval
The study was approved by the ethics committee of every participating institution and the Spanish Food and Drug Agency.
Informed consent
Every patient provided written or oral consent before being included in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
García-Donas, J., de Velasco, G., Madurga, R. et al. Case–control study assessing the impact of COVID19 in advanced kidney cancer patients treated with antiangiogenics or immunotherapy: the COVID-REN study. Clin Transl Oncol 26, 732–738 (2024). https://doi.org/10.1007/s12094-023-03295-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03295-2