Abstract
Renal cancer is the seventh most common cancer in men and the tenth in women. The aim of this article is to review the diagnosis, treatment, and follow-up of renal carcinoma accompanied by recommendations with new evidence and treatment algorithms. A new pathologic classification of RCC by the World Health Organization (WHO) was published in 2022 and this classification would be considered a “bridge” to a future molecular classification. For patients with localized disease, surgery is the treatment of choice with nephron-sparing surgery recommended when feasible. Adjuvant treatment with pembrolizumab is an option for intermediate-or high-risk cases, as well as patients after complete resection of metastatic disease. More data are needed in the future, including positive overall survival data. Clinical prognostic classification, preferably IMDC, should be used for treatment decision making in mRCC. Cytoreductive nephrectomy should not be deemed mandatory in individuals with intermediate-poor IMDC/MSKCC risk who require systemic therapy. Metastasectomy can be contemplated in selected subjects with a limited number of metastases or long metachronous disease-free interval. For the population of patients with metastatic ccRCC as a whole, the combination of pembrolizumab–axitinib, nivolumab–cabozantinib, or pembrolizumab–lenvatinib can be considered as the first option based on the benefit obtained in OS versus sunitinib. In cases that have an intermediate IMDC and poor prognosis, the combination of ipilimumab and nivolumab has demonstrated superior OS compared to sunitinib. As for individuals with advanced RCC previously treated with one or two antiangiogenic tyrosine-kinase inhibitors, nivolumab and cabozantinib are the options of choice. When there is progression following initial immunotherapy-based treatment, we recommend treatment with an antiangiogenic tyrosine-kinase inhibitor. While no clear sequence can be advocated, medical oncologists and patients should be aware of the recent advances and new strategies that improve survival and quality of life in the setting of metastatic RC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma (RCC) constitutes 80% of all primary renal neoplasms. The incidence remains stable in recent years with 431,288 new cases and 179,368 deaths in 2020 according to data published by GLOBOCAN. In Spain, the estimated incidence in 2022 was 8078 new cases (5572 in men and 2506 in women). It is twice as common in males and the median age at diagnosis is 64 years. Known risk factors include smoking, obesity, and hypertension. It is more prevalent among people with chronic renal failure, dialysis, renal transplant recipients, or those with tuberous sclerosis. Two percent are hereditary, normally Von Hippel-Lindau disease. Patients with bilateral tumors or characteristic alterations should be tested for germline mutations [1,2,3]
Diagnosis
Most tumors of the kidney (60%) were diagnosed incidentally by radiologic procedures performed for other medical indications [4]. The classic triad of flank pain, visible haematuria, and palpable abdominal mass is rare (6–10%) and correlates with aggressive histology, advanced disease, and worse outcomes [5]. Nonetheless, RCC is still the “internist’s cancer,” with paraneoplastic syndromes, such as hypercalcemia, unexplained fever, or erythrocytosis in approximately 30% of all cases [6]. Germline mutation testing and genetic counseling should be contemplated for younger patients (≤ 46 years) with RCC [7].
A physical examination should be performed together with a complete medical history. Laboratory tests include complete blood cell count, lactate dehydrogenase (LDH), serum creatinine, liver function study, serum-corrected calcium, and urinalysis [8].
Diagnosis is typically suggested by abdominal ultrasound, albeit an abdominal computed tomography scan (CT) is the gold standard to assess local invasiveness, venous involvement, locoregional lymph node involvement, or distant metastases. Nevertheless, it reveals poor differentiation between solid masses, fat-poor angiomyolipoma, and oncocytoma [9]. CT sensitivity for small renal masses surpasses 90%, approaching 100% for lesions > 2 cm [10]. In the case of a solid renal mass, the key criterion for malignant lesions is the presence of contrast enhancement or restriction. CT perfusion imaging detects temporal changes in tissue attenuation. It can pick up changes at the molecular level and evaluate tissue perfusion and vascular permeability. Perfusion studies are an indirect predictor of neoangiogenesis [11] and sensitivity and specificity to predict RCC were 100%, and 66.7%, respectively [12]. A chest CT is recommended, except in cT1a renal tumors, for which the probability of a positive chest CT is low [13].
Magnetic resonance imaging (MRI) is useful when evaluating local invasion and inferior vena cava involvement is suspected, or in case of allergy to the CT contrast agent or renal insufficiency. Routine bone or brain imaging is not indicated [14]. Bone scans can be performed if the subject has elevated serum alkaline phosphatase or reports bone pain. Cerebral CT or MRI will be carried out if clinical signs and symptoms point to brain metastases.
Fluorodeoxyglucose (FDG) Positron Emission Tomography (PET) is not currently regarded to be the standard imaging modality for the diagnosis of renal cancer [15], given its low sensitivity. As FDG is excreted by the kidneys, FDG PET is not suitable for local staging of primary RCC, but can be useful in metastatic RCC and to evaluate response to therapy. Although FDG is the most widely used tracer for PET scans, other new tracers are being studied, such as 68 Ga-PSMA, 18Ffluoroethylcholine,11C-acetate, 18F-fluoromisonidazole, and 18F-fluorothymidine [16, 17]. A renal tumor core biopsy provides high sensitivity (86–100%) and specificity (98–100%) to histopathologically confirm malignancy [18]. Needle core biopsies are preferable over fine needle aspiration for solid renal masses (RMs) [19], and is especially recommended prior to ablative therapies, as well as in patients with advanced disease before initiating systemic treatment [20].
Recommendations
-
CT scan is the gold standard for RCC staging. Level of evidence: III. Grade of recommendation: A.
-
Abdominal MRI is an alternative in various circumstances. Level of evidence: III. Grade of recommendation: C.
-
Neither bone scans nor brain CT (nor MRI) are recommended for routine clinical practice. Level of evidence: III. Grade of recommendation: D.
-
In patients without previous tumor diagnosis, a renal tumor core biopsy is recommended before treatment with ablative therapies, as well as in cases of metastatic disease, prior to starting systemic treatment. Level of evidence: III. Grade of recommendation: A.
Pathological and molecular classification
Renal cell carcinoma (RCC) is a heterogeneous disease. Histological classification is based on the tumor origin from different cells located in nephron cells but is completed with molecular and clinical information. The most common type of RCC is clear-cell renal cell carcinoma (ccRCC), accounting for up to 75% of all RCCs. Other major types include papillary (10–15%), chromophobe (5%), oncocytic (< 5%), Xp11 translocation (< 1%), or collecting-duct carcinomas (< 1%) [23]. There is a new pathology classification of RCC by the World Health Organization (WHO), 5th edition, published in 2022 (Table 3). We are witnessing an important change in papillary RCC pathological classification. No distinction is made between subtypes 1 and 2. Molecular research suggests that type 2 papillary RCC may not, in fact, constitute a truly independent entity [24]. New molecularly defined RCC subtypes are named and described: Eosinophilic solid and cystic RCC, elonging C (ELOC)-mutated RCC (formerly denominated Transcription elongation factor B (TCEB1)-mutated RCC), ALK-rearranged RCC, SMARCB1-deficient medullary RCC, TFEB-altered RCC, and fumarate hydratase (FH)-deficient RCC (formerly hereditary leiomyomatosis (HLRCC) syndrome-associated RCC. This classification “bridges” the present to a future molecular classification. Some entities are now regarded as independent types of RCC with specific clinical and molecular features, such as sporadic FH-deficient RCC, tubulocystic RCC, ESC RCC, clear cell papillary RCC, SMARCB1-deficient RCC, and microphthalmia-associated transcription factor (MiTF) family RCC [25].
Papillary RCC is considered the classical papillary RCC morphology type I, albeit certain single entities with papillary features can be regarded as variants of papillary RCC or provisional entities like papillary renal neoplasm with reversed polarity (PRNRP), biphasic hyalinizing psammomatous RCC (BHP RCC), biphasic squamoid/alveolar RCC, or thyroid-like follicular RCC (TLF RCC) Some have a specific molecular driver alteration, for example, KRAS mutations in PRNRP, NF2 mutations in BHP RCC, and EWSR1-PATZ1 fusions in TLF RCC.
While certain entities with eosinophilic or oncocytic cytoplasm are well defined, such as SDH-deficient RCC, ESC RCC, and FH-deficient RCC, others are emerging entities, for instance, eosinophilic vacuolated tumor (EVT) and low-grade oncocytic tumor (LOT). TSC mutations are important and are common in ESC RCC; similarly, TSC1/2 mutations or activating mTOR mutations have also been identified in EVT and LOT [25].
The differential diagnosis between cromophobe RCC and oncocytoma is essential. Chromophobe RCCs exhibit diffuse positivity for cytokeratin 7 (CK7), whereas oncocytomas are negative or present focal positivity for CK7. Molecular studies provide a more definitive diagnosis of subtypes.
ChRCC is characterized by chromosomal aneuploidy, TP53, PTEN, and mitochondrial gene mutations [26]. Collecting duct carcinoma (CDC), or Bellini duct carcinoma, continues to be a highly aggressive RCC arising from the renal collecting tubules or distal convoluted tubule. Recent NGS research has highlighted genomic alterations in SETD2, CDKN2A, SMARCB1, and NF2, and, together with transcriptomic studies, has confirmed the differences between urothelial carcinoma and CDC. Moreover, these tumors are characterized by having enriched tumor-infiltrating lymphocytes [27].
Sarcomatoid features are present in < 10% of RCC tumor and are mainly observed in patients with predominant clear cell areas [28]. Mutations in the gene encoding von Hippel–Lindau (VHL) disease tumor suppressor that leads to stabilization of hypoxia-inducible factor (HIF) are present in most ccRCCs (sporadic and hereditary forms). Loss of VHL function results in an upregulation of angiogenesis [29]. The Cancer Genome Atlas (TCGA) performed a comprehensive analysis of more than 400 ccRCC tumors that exhibited other mutated genes. The VHL gene was mutated in nearly 90% of the patients, while mutations that modify the chromatin-remodeling complex (PBRM1, ARID1A, and SMARCA4) and other epigenetic regulators, such as SETD2 and BAP1, are also commonly found [29, 30]. Although distinct histology tumor subtypes and molecular subtypes may associate varying sensitivity to therapies, validated predictive biomarkers are not available for clinical use [31].
Local and locoregional disease
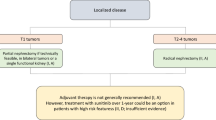
Surgery is the treatment of choice in stages I to III.
In tumors smaller than 7 cm (T1) the recommended treatment is partial nephrectomy (via open, laparoscopic or robot-assisted laparoscopic approaches), a technique that enables similar results to be achieved with better preservation of renal function [32]. Radical nephrectomy is an alternative if partial nephrectomy is not possible. Ablative procedures are options for elderly patients or those with high surgical risk, and in cases of multiple bilateral tumors, such as hereditary RCC, especially in small tumors. Renal biopsy is recommended if surgery is not possible [33]. Active surveillance is an option in elderly patients with significant comorbidities or short life expectancy and solid tumors < 4 cm [34].
In T2 tumors measuring > 7 cm, laparoscopic radical nephrectomy is the treatment of choice, while open surgery is called for in T3 and T4 tumors, albeit laparoscopic surgery can be contemplated in certain situations. Lymphadenectomy and suprarenalectomy are not indicated if there is no evidence of invasion on imaging tests(1), although the latter should be considered in upper pole tumors > 4 cm or > T3 [35].
The evidence regarding the treatment of venous thrombus is based on retrospective studies and poses a challenge not exempt of complications. Surgical intervention should be evaluated when feasible, as it may be associated with prolonged survival [36].
Adjuvant treatment
Until recently, several studies with angiogenesis and mTOR inhibitors had failed to demonstrate an overall survival benefit [37,38,39,40,41,42]; however, only one of the studies (in which sunitinib (S-TRAC) was administered) had demonstrated a disease-free survival benefit.
More recently, the results of a phase III trial have been published (Keynote-564), in which 994 intermediate- or high-risk patients (pT2 tumors with grade 4 or sarcomatoid features; pT3, any grade, node-negative tumors; pT4, any grade, node-negative tumors; any pT, any grade, node-positive tumors, or stage M1 with NED (defined as resection of the primary tumor and solid, isolated, soft-tissue metastases) were randomized to receive pembrolizumab vs. placebo for 1 year [43]. A benefit in disease-free survival (primary endpoint of the study) was demonstrated at 24 months (77.3% vs. 68.1%; HR 0.68; 95% CI 0.53–0.87; P = 0.002). A non-statistically significant trend toward a benefit was seen in overall survival in the pembrolizumab arm (HR 0.54, 95% CI 0.30–0.96, P = 0.0164).
Neoadjuvant treatment should be deemed experimental, in as much as it has only been studied in small trials.
Recommendations
-
Partial nephrectomy is recommended in T1 tumors, as well as in bilateral tumors or in patients with only one functioning kidney. Level of evidence: I. Grade of recommendation A.
-
Radical nephrectomy is recommended in T2-4 tumors. Level of evidence: II. Grade of recommendation: A.
-
Treatment with adjuvant pembrolizumab is an option for intermediate- or high-risk patients, as well as after complete resection of oligometastatic disease. More data are required in the future, including positive overall survival data. Level of evidence: I. Grade of recommendation: C.
-
Surgical intervention should be contemplated when feasible, as it may be associated with prolonged survival. Level of evidence: III.
Clinical and genomic prognostic classifications
The Memorial Sloan Kettering Cancer Center classification was formerly used for cytokine therapy [44]. Currently, the International Metastatic RCC Database Consortium (IMDC), based on a cohort of 645 patients and subsequently validated, constitutes the gold-standard prognostic assessment model for systemic therapy for metastatic RCC. The IMDC system includes six validated prognostic factors: Time from diagnostic to systemic therapy < 1 year; Karnofsky performance status (PS) < 80%; hemoglobin level below the lower limit of normal; corrected calcium above the upper limit of normal; neutrophils above the upper limit of normal; platelets above the upper limit of normal. The model classifies patients as good (0 factors), intermediate (1–2 factors), and poor (3–6 factors) risk [45, 46]. It has been validated in various pivotal randomized trials and is also predictive in second and successive lines of treatment and in non-clear cell RCC [47].
PD-L1 status has been studied to determine both its prognostic and predictive role. A meta-analysis appeared to establish the negative prognostic value of higher levels of expression of PD-L1 [48]. As for its possible predictive role, contradictory results have been reported in two meta-analyses, including several first-line, immuno-oncology (IO) combinations randomized trials [49, 50]. Considering all information, PD-L1 is not ready for routine use in clinical practice.
In recent years, several molecular models have been studied with the aim of identifying a predictive value. Beuselinck et al. identified four molecular subgroups with different sensitivity to sunitinib. The ones that associated better outcomes were ccrcc2 and ccrcc3 [51]. These subtypes were tested in a prospective, phase II trial with interesting results. Several prospective trials with IO combinations have inspired the intention to identify predictive molecular subgroups. In IMmotion150, comparing atezolizumab/bevacizumab with atezolizumab or sunitinib and following atezolizumab or sunitinib, angiogenic and T-effector profiles correlated with different outcomes depending on therapy [52]. The JAVELIN Renal 101 trial compared avelumab + axitinib vs. sunitinib and the authors identified a 26-gene immune signature and an angiogenesis signature with a different survival benefit for each treatments [53]. Moreover, seven molecular subgroups were identified among the IMotion151 sample, proposing different activity profiles [54]. On the other hand, in the CheckMate 214 trial, an association between inflammatory genes and survival was reported for IO combination [55, 56]. Nevertheless, none of these can be considered to possess any prognostic value.
To date, other biomarkers in tumor tissue, such as tumor-infiltrating immune cells or tumor mutation burden, as well as circulating biomarkers, such as circulating DNA, soluble PD-L1, or cytokines and inflammatory markers, have failed to demonstrate a definitive prognostic or predictive role. In a recent communication, the presence of CD8 + T-cell tumor infiltration correlates with a lack of favorable PBRM1 mutations, suggesting that there is a correlation between immune phenotypes and somatic alterations [57].
Recommendations:
-
Clinical prognostic classification, preferably IMDC, should be used for treatment decision-making in mRCC. Level of evidence: I. Grade of recommendation: B.
-
Genomic classification, including PD-L1 study, should not be used as a prognostic or predictive tool for treatment decision-making in mRCC. Level of evidence: II. Grade of recommendation: C.
Role of surgery in advanced renal cell carcinoma
The role of surgery in advanced disease is limited to cytoreductive nephrectomy, metastasectomy in oligometastatic disease, and palliative nephrectomy due to symptoms.
The role of cytoreductive nephrectomy has been modified based on improvements in systemic treatments.
At the time of interferon immunotherapy, two randomized studies comparing nephrectomy followed by interferon alpha versus interferon alpha established the role of upfront cytoreductive nephrectomy after demonstrating an overall survival benefit [58, 59].
At the time of antiangiogenic monotherapy, two randomized studies evaluated the role and timing of nephrectomy in advanced disease [60,61,62]. Using a non-inferiority design, the CARMENA study compared upfront nephrectomy followed by sunitinib with sunitinib alone in patients with intermediate and poor prognosis according to MSKCC criteria. The primary endpoint was median overall survival (18.4 months for sunitinib vs 13.9 months with surgery plus sunitinib; HR 0.89, 95% CI 0.71–1.10), thereby meeting the non-inferiority target (upper limit < 1.20)0.60 In a follow-up analysis, it appears that cytoreductive nephrectomy may be beneficial for patients with a single risk factor [61]. The SURTIME study sought to compare upfront nephrectomy followed by sunitinib and delayed nephrectomy after three cycles of sunitinib, but closed prematurely due to poor recruitment. The primary endpoint was progression-free survival for delayed nephrectomy (32 months vs 15 months), whereas one of the secondary endpoints was overall survival. However, the study’s poor accrual (fewer than 100 patients) meant that overall survival was the object of exploratory analysis [62]. After these two studies, upfront cytoreductive nephrectomy could be recommended for patients with 0–1 risk factors and good overall status, while those with ≥ 2 risk factors should be put on systemic treatment and nephrectomy should be reserved for those whose response to such treatment is very good.
Some retrospective studies have assessed the role of cytoreductive nephrectomy. Two of them, in particular, are particularly salient. The National Cancer Database and the IMDC. The first analyzed 15,390 patients identified in the National Cancer Database who were treated between 2006 and 2013 and revealed that overall survival was significantly better in nephrectomized patients (35%; 17.1 months vs 7.7 months) [63]. The second study relied on the IMDC to identify, 4639 patients eligible to participate in the study. It, too, concluded that there is an overall survival benefit for nephrectomized versus non-nephrectomized patients and that said benefit does not differ whether they receive antiangiogenic therapy or immunotherapy [64].
There are no prospective studies with current immunotherapy evaluating the role of nephrectomy; thus, physicians are recommended to follow the recommendations put forth during the era of antiangiogenic therapy while we await the results of the ongoing studies NORDIC-SUN, CYTOSHRINK, and PROBE.
As for metastasectomy in oligometastatic disease, there are different scenarios in which surgery can play a role, i.e., in subjects who present with synchronous oligometastatic (recommended when there are < 3 metastases), metachronous (disease-free interval > 1 year), or residual disease following a good response to systemic treatment. The strongest predictors of prolonged survival were a disease-free interval from nephrectomy to metastases > 1 year, ≤ 2 vs > 2 sites (especially if the lung was involved as opposed to the brain), ECOG 0–1, and absence of sarcomatoid features [65]. Different forms of radiotherapy can also be considered as local treatment in selected cases.
Recommendations
-
Debulking or cytoreductive nephrectomy should not be deemed mandatory in patients with intermediate-poor IMDC/MSKCC risk who require systemic therapy. Level of evidence: I. Grade of recommendation: A.
-
Cytoreductive nephrectomy may play a role in the management of advanced renal cell carcinoma in individuals with limited metastatic burden amenable to surveillance or metastasectomy, in patients requiring palliation, and potentially delayed cytoreductive nephrectomy in patients with a favorable response or stable disease after initial systemic therapy. Level of Evidence: II. Grade of recommendation: B.
-
Metastasectomy can be contemplated in selected patients having a limited number of metastases or long metachronous disease-free interval. Level of evidence: II. Grade or recommendation: C.
First-line systemic therapy for metastatic clear cell RCC (mccRCC)
Metastatic ccRCC includes a variety of poorly understood clinical situations with different prognoses, including patients with indolent oligometastatic relapses, and others with very aggressive disease associated with short survival. On the other hand, most therapies available exert their effect by acting on the microenvironment instead of the tumor cells themselves.
For many decades, the only active treatments were non-specific immunotherapies (interferon and IL-2) that were associated with high toxicity and 10% long-term survival in a selected subgroup of patients [66].
Following the discovery that RCC is closely associated with the loss of VHL gene activity, with increased VGEF and growth factors, angiogenesis, and anaerobic metabolism, a number of antiangiogenic tyrosine-kinase inhibitors (TKI) against the endothelium VGEF receptor (VEGFR) were developed and examined in phase III trials. These drugs exhibited a statistically significant increase in PFS against interferon (sunitinib [67]), placebo (pazopanib [68, 69]), and sorafenib (tivozanib [70]). TKIs achieved a median PFS of approximately 8–11 months and a median OS approaching 2 years, becoming the standard first-line treatment for the overall population of patients with metastatic ccRCC. These results varied according to the prognostic subgroups described in the IMDC classification, which was specifically developed for patients with metastatic ccRCC treated with antiangiogenic TKIs or bevacizumab. TKIs were particularly effective for patients in the “good prognostic group”, who achieved a median OS of 43 months at a time when effective salvage treatments were not yet available [71]. Even in this subgroup, some authors recommend local treatment of metastases and/or close follow-up for selected, asymptomatic, well-informed patients with the oligometastatic disease having a small tumor burden [72]. Finally, another VEGFR-TKI, cabozantinib, improved ORR and PFS compared with sunitinib in a randomized phase II trial in subjects with intermediate and poor risk [73], while the mTOR inhibitor (temsirolimus) was associated with longer OS versus interferon in a population of patients having a poor prognosis [74].
Recently, immune check point inhibitors (ICIs) promoting antigen presentation (Ipilimumab) or blocking antiPDL1/PD1 signaling to reverse tumor immune evasion mechanisms (atezolizumab, avelumab, nivolumab or pembrolizumab) have been developed against RCC. Due to the poor results achieved with TKIs in the intermediate and poor IMDC subgroups, the Checmate-214 phase III trial compared the combination of Ipilimumab-Nivolumab versus sunitinib in these subgroups and detected higher ORR and OS with the combination therapy [75]. After a median follow-up of 42 months, the median OS was 47 vs 26.6 months (HR: 0.66) and ORR 42% (11% CR) vs 26% (2% CR) in favor of the combination of drugs; in addition, a plateau in PFS was observed at approximately 35% after 2 years in both study groups [76]. A post hoc analysis suggested that the OS benefit might persist in patients who discontinued therapy due to adverse side effects. After these results, the combination of Ipilimumab-Nivolumab was considered standard for patients with IMDC intermediate-poor prognosis. In an exploratory analysis, no specific benefit was seen in the good-risk population.
Several combinations of ICIs and TKI anti-VEGFR have been compared with sunitinib in phase III trials. The IMmotion 151 trial [77], demonstrated a 3.5-month increase in PFS with atezolizumab-bevacizumab in the PDL1 + subgroup, but not in OS in the overall population [78]. Likewise, no significant increase in OS has been reported in the JAVELIN renal 101 trial with avelumab-axitinib, despite increased PFS in both the PDL1 + treatment arm and the overall study population [79].
However, three trials have clearly demonstrated the statistically significant superiority of ICI-TKI combinations over sunitinib in ORR, PFS, and OS in the whole population of metastatic ccRCC. In the Keynote 426 study (pembrolizumab + axitinib vs sunitinib) [80], ORR was 59% vs 36%, median PFS 15.1 vs 11.1 months (HR 0.69), and median OS was not reached vs 35.7 months (HR: 0.53). In the CheckMate 9ER trial (nivolumab + cabozantinib vs sunitinib) [81], ORR was 56% vs 27%, median PFS 16.6 vs 8.3 months (HR: 0.51), and median OS was not reached in either group (HR: 0.60). Finally, the CLEAR trial (lenvatinib + pembrolizumab vs sunitinib) demonstrated an ORR of 71% vs 36%, with median PFS of 24 vs 9 months (HR: 0.39), and median OS was not reached in either group (HR: 0.66) [82]. All of these trials have consistently reported up to 70% grade 3 or higher adverse events of any cause both groups that are usually manageable, although as many as 5–20% of the participants discontinued of at least one of the drugs. Despite clear evidence of efficacy with the above combinations, certain areas of uncertainty persist. First, it should be noted that there are no definitive predictive factors allowing the medical oncologist to choose the most appropriate treatment for each patient. Second, indirect comparations among the ICI-TKI combinations are methodologically flawed given the differences in the baseline characteristics of the populations included in each trial. Third, all of these trials were designed to test hypothesis on the entire population of patients with metastatic ccRCC. Despite all the trials being stratified by IMDC or MSKCC prognostic classifications, subgroup analyses should be regarded as exploratory. More specifically, some uncertainty may arise with respect to the IMDC good prognosis subgroup, where none of the combinations obtained a clear advantage in terms of OS, despite the fact that some combinations displayed a higher ORR and longer PFS over sunitinib in the subgroup analysis. On the other hand, exploratory analyses of these trials (including Checkmate 214) invariably exhibited an advantage in OS over sunitinib in the subgroup with sarcomatoid differentiation (Fig. 1). Finally, an improvement in PFS has been reported recently with the triple combination of ipilimumab – nivolumab – cabozantinib vs sunitinib in the COSMIC-313 trial; nevertheless, OS results are still pending [83].
Recommendations
Until predictive factors became reliable, the choice of first-line treatment for patients with metastatic ccRCC should be based on the local availability of approved drugs, patient comorbidities and prognosis, including the need for a quick response, as well as the design of a global therapeutic strategy with salvage options for subjects who do not respond or who relapse (Fig. 1).
Considering the whole population of patients with metastatic ccRCC:
-
The combination of pembrolizumab + axitinib, nivolumab + cabozantinib, or pembrolizumab + lenvatinib can be considered the first options based on the benefit obtained in OS over sunitinib. Level of evidence: I. Grade of recommendation: A.
-
Given its superiority on PFS over sunitinib, the combination of avelumab + axitinib is an alternative when other combinations are not available. Level of evidence: I. Grade of recommendation: B.
-
Sunitinib, pazopanib, and tivozanib are reasonable options when the above-mentioned combinations are not available. Level of evidence: I. Grade of recommendation: B.
In the context of an individualized decision-making based on IMDC subgroups, in addition to the above recommendations:
-
In patients with IMDC intermediate or poor prognosis, Ipilimumab + nivolumab should also be considered standard, given the benefit observed in OS over sunitinib. Level of evidence: I. Grade of recommendation: A. In this subpopulation, cabozantinib could be preferable to sunitinib based on the longer PFS obtained in a randomized phase II study. Level of evidence: I. Grade of recommendation: C Although not widely used, temsirolimus remains an option for poor-risk IMDC patients. Level of evidence: I. Grade of recommendation: C.
-
No definitive evidence is available as to the benefit of the anti PD1/PDL1 plus either ipilimumab or TKI over sunitinib alone in the IMDC favourable subgroup. For asymptomatic patients with indolent and good-prognosis disease, active surveillance can be considered. Level of evidence: II. Grade of recommendation: C.
Second-line treatment and sequences
In the past, the standard of care in first line was a TKI in monotherapy and many patients are still being treated with VEGF in monotherapy today. The anti-PD1 inhibitor nivolumab [84, 85] or the oral TKI targeting VEGFR, MET, and AXL VEGFR-TKI cabozantinib [86, 87] are the standard treatment for patients progressing to a previous anti-VEGF treatment. Both trials demonstrated increased OS and PFS as compared to everolimus. The combination of lenvatinib (another anti-VEGFR1-3, -FGFR, and -PDGFR oral TKI) with everolimus [88] exhibited improved PFS over everolimus in a randomized phase II trial in this population.
With the advent of first-line IO-based combinations, the therapeutic sequence in mRCC has changed and prospective randomized studies exploring what to do after failure of IO-based combinations are lacking. Furthermore, the phase III TIVO-3 trial [89] revealed greater PFS associated with tivozanib compared to sorafenib in a heavily pretreated population.
Several retrospective series have investigated the usefulness of multiple tyrosine kinase inhibitors (sunitinib, pazopanib, axitinib, cabozantinib, lenvatinib-everolimus) after IO treatment [90,91,92,93,94,95,96,97,98,99,100]. On the whole, these series displayed an ORR between 20–54% and PFS or TTF ranging from 6 to 13 months.
Prospective data exploring single-agent activity cabozantinib comes from a post hoc analysis of the METEOR trial. Of 32 patients who had received prior IO therapy (5% of the total cohort), activity favored cabozantinib over everolimus consistent with the overall population, median PFS (HR, 0.22), and a trend in overall survival with an ORR of 22% versus 0% [101]. A post hoc analysis from the TIVO-3 study, in which tivozanib was compared to sorafenib as third or fourth-line therapy in mRCC, also investigated the usefulness of tivozanib post-IO. Of 350 patients, 91 (26%) had previously received treatment with IO; median PFS favored tivozanib at 7.3 months compared to 5.1 months with sorafenib (hazard ratio 0.55). The overall response rate was not reported in this cohort.
Prospective data have also been published from small phase II trials with axitinib [102], cabozantinib [103], and sunitinib [104]. A phase II trial with dose escalation of axitinib was carried out in 40 patients who had received checkpoint inhibitor therapy as the most recent treatment. There was no limit on the number of previous therapies and 70% had received prior VEGF therapy. The trial reported 8.8 month PFS and an ORR of 45% [102]. In the Breakpoint trial, 22 patients were treated with cabozantinib after adjuvant or first-line PD-1/PD-L1-based therapy (as monotherapy or in combination). The primary endpoint was PFS, which was 9.3 months and the ORR was 43% [103]. In the Immunosun trial, subjects who had progressed following a first-line regimen consisting of an ICI-based therapy were treated with sunitinib. PFS and ORR were 5.6 months and 19%, respectively [104].
Recommendations
-
In patients with advanced RCC previously treated with one or two antiangiogenic tyrosine-kinase inhibitors, nivolumab, and cabozantinib are the recommended options. Level of evidence: I. Grade of recommendation: A. Decisions to use either agent may be based on the expected toxicity and on contraindications for each drug, as randomized data is lacking.
-
Axitinib, everolimus, lenvatinib + everolimus, and tivozanib are alternatives for second-line, providing that they are available and patients cannot receive nivolumab or cabozantinib. Level of evidence: I. Grade of recommendation: B. In addition, they may also be acceptable options following nivolumab and cabozantinib. Level of evidence: III. Grade of recommendation: C.
-
For patients who progress after initial immunotherapy-based treatment, we suggest treatment with an anti-VEGFR TKI. Options include cabozantinib, axitinib, tivozanib, sunitinib, and pazopanib. Further research is required in this context. Level of evidence: III. Grade of recommendation: C.
-
Patients should be encouraged to participate in clinical trials whenever possible.
Non-clear renal cell renal carcinoma.
Approximately, 15–20% of renal cell carcinomas (RCC) are classified as non-clear cell renal cell carcinoma (nccRCC), which are then further divided into multiple distinct subtypes based on histological and molecular characteristics. Subtypes of nccRCC include papillary, chromophobe, collecting duct, renal medullary, and translocation RCC, which account for 10–15%, 5–7%, 1–2%, < 1%, and < 1% of all RCCs, respectively [105]. All subtypes can have sarcomatoid differentiation. Median survival of individuals with localized nccRCC varies with histology, with more favorable outcomes in patients with papillary and chromophobe RCC. In the metastatic setting, however, survival in all subtypes of nccRCC is uniformly worse compared to ccRCC, due to the inherent aggressiveness of these cancers and a lack of effective systemic treatment options [106]. These patients have significantly lower RR and poorer mPFS and mOS than those with ccRCC (ORR 10.5%, mPFS 7.4 months, and mOS 13.4 months) [107]. Clinical data are limited in these rare histological subtypes, which tend to be excluded from controlled phase III trials, and most antitumor activity data are derived from retrospective studies, expanded access programmes, and prospective single-arm studies. The treatment of localized forms (stages I, II, and III) is comparable to ccRCC. There is no evidence of the efficacy of adjuvant treatment, as the majority of trials in the adjuvant setting did not include patients with nccRCC, and only two of them included a number too small as to enable any conclusions to be drawn. The role of cytoreduction in advanced disease is controversial, given that the CARMENA and SURTIME trials did not include nccRCC patients. Two retrospective series, the International Metastatic Database Consortium (IMDC) and the National Cancer Database (NCD) included 510 and 3201 patients, respectively, with advanced nccRCC tumors. Both showed a significant impact on OS in favor of the arm that included surgery (20.6 vs. 9.6 and 17.1 vs. 7.7 months, respectively) [107, 108]. Regarding metastasectomy, nccRCC patients are scarcely reflected in clinical trials and subgroup analyses are not available. Therefore, cytoreductive nephrectomy and/or metastasectomy would be an option in some patients, and the decision must be made on a case-by-case basis. The first prospective data in advanced disease were collected from a subset analysis of the phase III study of temsirolimus, which allowed non-clear-cell renal cell carcinoma and exhibited comparable efficacy with the clear-cell renal cell carcinoma cohort [109]. Three subsequent randomized phase II trials comparing sunitinib to everolimus included a diverse array of non-clear histologies and provided evidence that front-line sunitinib induces better PFS, albeit to a modest degree, when compared with PFS observed in clear-cell disease [110,111,112]. These results were further supported by data from expanded access programs. A meta-analysis was performed that included 365 patients with advanced nccRCC. The pooled HR for PFS was 1.30 (95% CI 0.91–1.86, p = 0.15), indicating a trend toward superiority of sunitinib over everolimus, although the results failed to reach statistical significance [113]. Other anti-VEGFR tyrosine kinase inhibitors, such as axitinib and pazopanib, have been evaluated in small prospective series and retrospective analyses that have yielded promising activity comparable to that achieved in the clear-cell population [114,115,116]. Nevertheless, most of these studies only patients enrolled subjects with papillary and chromophobe tumors. A small retrospective study and real-world data from an Italian expanded access program have reported moderate efficacy with cabozantinib, revealing overall response rates of 23%, mPFS of 8 months, and mOS of 12 months [117, 118]. As the majority of the papillary-specific studies investigated the use of c-MET inhibition, due to the increased incidence of alterations in the MET proto-oncogene in these tumors [119], cMET inhibitors such as cabozantinib appear to represent an acceptable option instead of the usual anti-VEGF TKIs. The safety and efficacy of immune checkpoint inhibitors (ICIs) have been explored in nccRCC in the KEYNOTE-427 study of pembrolizumab, a subgroup analysis of the CheckMate 374 study of nivolumab, and an expanded access program for nivolumab [120,121,122]. Additionally, a phase II trial of atezolizumab and bevacizumab included patients with nccRCC and clear cell renal cell carcinoma with sarcomatoid differentiation (sccRCC) [123]. Most of the patients had papillary RCC. ORRs ranged from 13 to 26%. Data from prospective studies with combinations of ICIs and TKIs are also available. In the CALYPSO phase I/II trial utilizing the combination of savolitinib + durvalumab, an ORR of 29% was achieved in 41 patients with papillary renal cell carcinoma (up to 40% in MET-driven tumors) [124, 125]. Cabozantinib and nivolumab have displayed activity as well in 47 nccRCC patients with different histologies, with an ORR of 47.5% in papillary, unclassified, and translocation-associated RCC. In addition to these data, sarcomatoid histologies, tumors of the collecting ducts, and medullary renal carcinoma have traditionally been contemplated as benefitting from chemotherapy. Two series present their role in sarcomatoid histology, yielding a modest benefit in these patients. Some data suggest that sarcomatoid tumors are highly inflamed tumors that usually associate poor-risk features and are sensitive to ICIs. Nevertheless, there are no randomized clinical trials available to confirm the efficacy of immunotherapy in first-line treatment [126, 127]. Collecting-duct tumors tend to be resistant to systemic therapy. Nonetheless, platinum-based chemotherapy is usually recommended based on small prospective and retrospective series. After first-line therapy, there are insufficient data to allow any recommendation to be made. However, at least for papillary tumors, which are the most common non-ccRCCs, the use of the ccRCC algorithm is an acceptable option.
Recommendations
-
Clinical data are limited in nccRCC, which are usually excluded from controlled phase III trials. Therefore, enrolment into specific clinical trials is strongly recommended. Level of evidence: V. Grade of evidence: A.
-
There are no available data regarding post-nephrectomy adjuvant treatment in localized nccRCC.
-
In the first-line setting, the most robust data exist for sunitinib, although other targeted therapies, such as TKI and mTOR have limited data. While specific data are not available, the choice of treatment should be based on each specific subtype:
-
Papillary: Sunitinib: Level of evidence: II. Grade of evidence: B. Pazopanib: Level of evidence: III. Grade of evidence: C. Everolimus: Level of evidence: II. Grade of evidence: C. Cabozantinib: Level of evidence: IV. Grade of evidence: c.
-
Cromophobe: Sunitinib: Level of evidence: II. Grade of evidence: C. Pazopanib: Level of evidence: III. Grade of evidence: C. Everolimus: Level of evidence: II. Grade of evidence: C.
-
Collecting duct/Medullary: Cisplatin or carboplatin- based regimen: Level of evidence: III. Grade of evidence: C.
-
Sarcomatoid: Sunitinib. Level of evidence: II. Grade of evidence: B. Pazopanib: Level of evidence: III. Grade of evidence: C. Nivolumab+ipilimumab: Level of evidence: IV. Grade of evidence: C.
-
-
After first-line, no recommendation is possible based on available data.
Methodology
This guideline has been developed based on the consensus of ten genitourinary medical oncologists, designed by the Spanish Society of Medical Oncology (SEOM) and the Spanish Oncology Genitourinary Group (SOGUG), and an external review panel comprising two experts designated by SEOM. The Infectious Diseases Society of America–US Public Health Service Grading System for Ranking Recommendations in Clinical Guidelines has been used to assign levels of evidence and grades of recommendation (Table 4).
Data availability
Not applicable.
References
Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245.
Global Cancer Observatory. International Agency for Research on Cancer. World Health Organization. Available at: https://gco.iarc.fr/ (Accessed on Dec 16, 2022)
https://seom.org/images/LAS_CIFRAS_DEL_CANCER_EN_ESPANA_2022.pdf. (Accessed on Dec 16, 2022).
Vasudev NS, Wilson M, Stewart GD, Adeyoju A, Cartledge J, Kimuli M, et al. Challenges of early renal cancer detection: symptom patterns and incidental diagnosis rate in a multicentre prospective UK cohort of patients presenting with suspected renal cancer. BMJ Open. 2020;10(5): e035938.
Lee CT, Katz J, Fearn PA, Russo P. Mode of presentation of renal cell carcinoma provides prognostic information. Urol Oncol. 2002;7(4):135–40.
Sacco E, Pinto F, Sasso F, Racioppi M, Gulino G, Volpe A, et al. Paraneoplastic syndromes in patients with urological malignancies. Urol Int. 2009;83(1):1–11.
Shuch B, Vourganti S, Ricketts CJ, Middleton L, Peterson J, Merino MJ, et al. Defining early-onset kidney cancer: implications for germline and somatic mutation testing and clinical management. J Clin Oncol. 2014;32:431–7.
Magera JS, Leibovich BC, Lohse CM, Sengupta S, Cheville JC, Kwon ED, et al. Association of abnormal preoperative laboratory values with survival after radical nephrectomy for clinically confined clear cell renal cell carcinoma. Urology. 2008;71(2):278–82.
Rossi SH, Prezzi D, Kelly-Morland C, Goh V. Imaging for the diagnosis and response assessment of renal tumours. World J Urol. 2018;36:1927–42.
Jamis-Dow CA, Choyke PL, Jennings SB, Linehan WM, Thakore KN, Walther MM. Small (<or = 3 cm) renal masses: detection with CT versus US and pathologic correlation. Radiology. 1996;198(3):785–8.
Tatar G, Alçin G, Samanci NS, Fenercioglu OE, Beyhan E, Çermik TF. Role of 18F-FDG PET/CT in the assessment of therapy response and clinical outcome in metastatic renal cell carcinoma treated with tyrosine kinase inhibitors or immunotherapy. Nucl Med Commun. 2022;43(6):701–9.
Mazzei FG, Mazzei MA, Cioffi Squitieri N, Pozzessere C, Righi L, Cirigliano A, et al. CT perfusion in the characterisation of renal lesions: an added value to multiphasic CT. Biomed Res Int. 2014. https://doi.org/10.1155/2014/135013.
Voss J, Drake T, Matthews H, Jenkins J, Tang S, Doherty J, et al. Chest computed tomography for staging renal tumours: validation and simplification of a risk prediction model from a large contemporary retrospective cohort. BJU Int. 2020;125:561–7.
Koga S, Tsuda S, Nishikido M, Ogawa Y, Hayashi T, Kanetake H. The diagnostic value of bone scan in patients with renal cell carcinoma. J Urol. 2001;166:2126.
Jena R, Narain TA, Singh UP, Srivastava A. Role of Positron Emission Tomography/computed tomography in the evaluation of renal cell carcinoma. Indian J Urol. 2021;37:125–32.
Aggarwal A, Das CJ, Sharma S. Recent advances in imaging techniques of renal masses. World J Radiol. 2022;14(6):137–50.
Urso L, Castello A, Rocca GC, Lancia F, Panareo S, Cittanti C, et al. Role of PSMA-ligands imaging in renal cell carcinoma management: current status and future perspectives. J Cancer Res Clin Oncol. 2022;148(6):1299–311.
Marconi L, Dabestani S, Lam TB, Hofmann F, Stewart F, Norrie J, et al. Systematic review and meta- analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol. 2016;69:660–73.
Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European association of urology guidelines on renal cell carcinoma: the 2022 Update. Eur Uroly. 2022;82(2022):399–410.
Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(5):706–20.
Brierley JD, Gospodarowicz M, Wittekind C, editors. UICC TNM classification of malignant tumours. 8th ed. New York: Wiley Black-well; 2016.
Delahunt B, Eble JN, Samaratunga H, Thunders M, Yaxley JW, Egevad L. Staging of renal cell carcinoma: current progress and potential advances. Pathology. 2021;53(1):120–8.
Kim WY. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004;22:4991–5004.
WHO. Classification of tumours of the urinary system and male genital organs. 5th ed. Lyon: International Agency for Research on Cancer; 2022.
Netto GJ, Amin MB, Berney BM, Compérat EM, Gill AJ, Hartmann A, et al. The 2022 World Health Organization classification of tumors of the urinary system and male genital organs-part B: prostate and urinary tract tumors. Eur Urol. 2022;82(5):469–82.
Garje R, Elhag D, Yasin HA, Acharya L, Vaena D, Dahmoush L. Comprehensive review of chromophobe renal cell carcinoma. Crit Rev Oncol Hematol. 2021;160: 103287.
Suarez C, Marmolejo D, Valdivia A, Morales-Barrera R, Gonzalez M, Mateo J, et al. Update in collecting duct carcinoma: current aspects of the clinical and molecular characterization of an orphan disease. Front Oncol. 2022;12: 970199.
Blum KA, Gupta S, Tickoo SK, Chan TA, Russo P, Motzer RJ, et al. Sarcomatoid renal cell carcinoma: biology, natural history and management. Nat Rev Urol. 2020;17(12):659–78.
Dizman N, Philip EJ, Pal SK. Genomic profiling in renal cell carcinoma. Nat Rev Nephrol. 2020;16(8):435–51.
Ricketts CJ, De Cubas AA, Fan H, Smith CC, Lang M, Reznik E, et al. The Cancer Genome Atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep. 2018;23(1):313–26.
Linehan WM, Ricketts CJ. The Cancer Genome Atlas of renal cell carcinoma: findings and clinical implications. Nat Rev Urol. 2019;16(9):539–52.
MacLennan S, Imamura M, Lapitan MC, Omar MI, Lam TBL, Hilvano-Cabungcal AM, et al. Systematic review of oncological outcomes following surgical management of localised renal cancer. Eur Urol. 2012;61(5):972–93.
Pierorazio PM, Johnson MH, Patel HD, Sozio SM, Sharma R, Iyoha E, et al. Management of renal masses and localized renal cancer: systematic review and meta-analysis. J Urol. 2016;196(4):989–99.
Mir MC, Capitanio U, Bertolo R, Ouzaid I, Salagierski M, Kriegmair M, et al. Role of active surveillance for localized small renal masses. Eur Urol Oncol agosto de. 2018;1(3):177–87.
Kuczyk M, Wegener G, Jonas U. The therapeutic value of adrenalectomy in case of solitary metastatic spread originating from primary renal cell cancer. Eur Urol agosto de. 2005;48(2):252–7.
Welz A, Schmeller N, Schmitz C, Reichart B, Hofstetter A. Resection of hypernephromas with vena caval or right atrial tumor extension using extracorporeal circulation and deep hypothermic circulatory arrest: a multidisciplinary approach. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 1997;12(1):127–32.
Motzer RJ, Ravaud A, Patard JJ, Pandha HS, George DJ, Patel A, et al. Adjuvant sunitinib for high-risk renal cell carcinoma after nephrectomy: subgroup analyses and updated overall survival results. Eur Urol. 2018;73(1):62–8.
Haas NB, Manola J, Uzzo RG, Flaherty KT, Wood CG, Kane C, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet Lond Engl. 2016;387(10032):2008–16.
Motzer RJ, Haas NB, Donskov F, Gross-Goupil M, Varlamov S, Kopyltsov E, et al. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(35):3916–23.
Gross-Goupil M, Kwon TG, Eto M, Ye D, Miyake H, Seo SI, et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Ann Oncol Off J Eur Soc Med Oncol. 2018;29(12):2371–8.
Eisen T, Frangou E, Oza B, Ritchie AWS, Smith B, Kaplan R, et al. Adjuvant sorafenib for renal cell carcinoma at intermediate or high risk of relapse: results from the SORCE randomized phase III intergroup trial. J Clin Oncol Off J Am Soc Clin Oncol. 2020;38(34):4064–75.
Ryan CW, Tangen C, Heath EI, Stein MN, Meng M, Alva AS, et al. EVEREST: Everolimus for renal cancer ensuing surgical therapy—a phase III study (SWOG S0931, NCT01120249). J Clin Oncol. 2022;40(17_suppl):LAB500–5001.
Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang YH, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med. 2021;385(8):683–94.
Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20(1):289–96.
Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–9.
Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141–8.
Ko JJ, Xie W, Kroeger N, Lee JL, Rini BI, Knox JJ, et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol. 2015;16(3):293–300.
Iacovelli R, Nolè F, Verri E, Renne G, Paglino C, Santoni M, et al. Prognostic role of PD-L1 expression in renal cell carcinoma. A systematic review and meta-analysis. Target Oncol. 2016;11(2):143–8.
Mori K, Abufaraj M, Mostafaei H, Quhal F, Fajkovic H, Remzi M, et al. The predictive value of Programmed Death Ligand 1 in patients with metastatic renal cell carcinoma treated with immune-checkpoint inhibitors: a systematic review and meta-analysis. Eur Urol. 2021;79(6):783–92.
Carretero-González A, Lora D, Martín Sobrino I, Sáenz Sanz I, Bourlon MT, Anido Herranz U, et al. The value of PD-L1 expression as predictive biomarker in metastatic renal cell carcinoma patients: a meta-analysis of randomized clinical trials. Cancers (Basel). 2020;12(7):1945.
Beuselinck B, Job S, Becht E, Karadimou A, Verkarre V, Couchy G, et al. Molecular subtypes of clear cell renal cell carcinoma are associated with sunitinib response in the metastatic setting. Clin Cancer Res. 2015;21(6):1329–39.
Vano YA, Elaidi R, Bennamoun M, Chevreau C, Borchiellini D, Pannier D, et al. Nivolumab, nivolumab-ipilimumab, and VEGFR-tyrosine kinase inhibitors as first-line treatment for metastatic clear-cell renal cell carcinoma (BIONIKK): a biomarker-driven, open-label, non-comparative, randomised, phase 2 trial. Lancet Oncol. 2022;23(5):612–24.
McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24(6):749–57.
Motzer RJ, Robbins PB, Powles T, Albiges L, Haanen JB, Larkin J, et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: biomarker analysis of the phase 3 JAVELIN Renal 101 trial. Nat Med. 2020;26(11):1733–41.
Motzer RJ, Powles T, Atkins MB, Escudier B, McDermott DF, Alekssev BY, et al. Final overall survival and molecular analysis in IMmotion151, a phase 3 trial comparing atezolizumab plus bevacizumab vs sunitinib in patients with previously untreated metastatic renal cell carcinoma. JAMA Oncol. 2022;8(2):275–80.
Motzer RJ, Choueiri TK, McDermott DF, Powles T, Vano YA, Gupta S, et al. Biomarker analysis from CheckMate 214: nivolumab plus ipilimumab versus sunitinib in renal cell carcinoma. J Immunother Cancer. 2022;10(3): e004316.
Braun DA, Hou Y, Bakouny Z, Ficial M, Sant’ Angelo M, Forman J, et al. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat Med. 2020;26(6):909–18.
Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, et al. Nephrectomy followed by interferon α2b compared with interferon α2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345(23):1655–9.
Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R, European Organisation for Research and Treatment of Cancer (EORTC) Genitourinary Group. Radical nephrectomy plus interferon-alpha-based immunotherapy compared with interferon alpha alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358(9286):966–9670.
Méjean A, Ravaud A, Thezenas S, Colas S, Beauval J-B, Bensalah K, et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med. 2018;379(5):417–27.
Méjean A, Ravaud A, Thezenas S, Chevreau C, Bensalah K, Geoffrois L, et al. Sunitinib alone or after nephrectomy in metastatic renal cell carcinoma: is there still a role for cytoreductive nephrectomy? Eur Urol. 2021;80(4):417–24.
Bex A, Mulders P, Jewett M, Wagstaff J, van Thienen JV, Blank CU, et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: the SURTIME randomised clinical trial. JAMA Oncol. 2019;5:164.
Hanna N, Sun M, Meyer CP, Nguyen PL, Pal SK, Chang SL, et al. Survival analyses of patients with metastatic renal cancer treated with targeted therapy with or without cytoreductive nephrectomy: a National Cancer Database Study. J Clin Oncol. 2016;34(27):3267–75.
Bakouny Z, El Zarif T, Dudani S, Connor Wells J, Gan CL, Donskov F, et al. Upfront cytoreductive nephrectomy for metastatic renal cell carcinoma treated with immune checkpoint inhibitors or targeted therapy: an observational study from the international metastatic renal cell carcinoma database consortium. Eur Urol. 2022. https://doi.org/10.1016/j.eururo.2022.10.004.
Kavolius JP, Mastorakos DP, Pavlovich C, Russo P, Burt ME, Brady MS. Resection of metastatic renal cell carcinoma. J Clin Oncol. 1998;16(6):2261–6.
McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(1):133–41.
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–24.
Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–8.
Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–31.
Motzer RJ, Nosov D, Eisen T, Bondarenko I, Lesovoy V, Lipatov O, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J Clin Oncol. 2013;31(30):3791–9.
Heng DY, Xie W, Regan MM, Harshnan LC, Bjarnason GA, Vaishampayan UN, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141–8.
Rini BI, Dorff TB, Elson P, Rodriguez CS, Shepard D, Wood L, et al. Active surveillance in metastatic renal-cell carcinoma: a prospective, phase 2 trial. Lancet Oncol. 2016;17(9):1317–24.
Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(6):591–7.
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–81.
Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90.
Tannir NM, McDermott DF, Escudier B, Hammers HJ, Aren OR, Plimack ER, et al. Overall survival and independent review of response in CheckMate 214 with 42-month follow-up: first-line nivolumab + ipilimumab (N+I) versus sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol. 2020;38(6_suppl):609–609.
Rini BI, Powles T, Atkins MB, Escudier B, McDemott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393(10189):2404–15.
Motzer RJ, Powles T, Atkins MB, Escudier B, McDemott DF, Alekseev BY, et al. Final overall survival and molecular analysis in IMmotion151, a phase 3 trial comparing atezolizumab plus bevacizumab vs sunitinib in patients with previously untreated metastatic renal cell carcinoma. JAMA Oncol. 2022;8(2):275–80.
Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–15.
Powles T, Plimack ER, Soulières D, Waddell T, Stus V, Gafanov R, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;2:1563–73.
Choueiri TK, Powles T, Burotto M, Escudier M, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384:829–41.
Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;8(384):1289–300.
Choueiri TK, Powles TB, Albiges L, Burotto M, Szczylik C, Zurawski B, et al. Phase III study of cabozantinib (C) in combination with nivolumab (N) and ipilimumab (I) in previously untreated advanced renal cell carcinoma (aRCC) of IMDC intermediate or poor risk (COSMIC-313). Ann Oncol. 2022;33(suppl_7):S808–69.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13.
Motzer RJ, Escudier B, George S, Hammers HJ, Srinivas S, Tykodi SS, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer. 2020;126(18):4156–67.
Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1814–23.
Choueiri TK, Escudier B, Powles T, Tannir MM, Mainwaring PN, Rini BI, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):917–27.
Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16(15):1473–82.
Rini BI, Pal SK, Escudier BJ, Atkins MB, Hutson TE, Porta C, et al. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020;21(1):95–104.
Nadal R, Amin A, Geynisman DM, Voss MH, Weinstock M, Doyle J, et al. Safety and clinical activity of vascular endothelial growth factor receptor (VEGFR)-tyrosine kinase inhibitors after programmed cell death 1 inhibitor treatment in patients with metastatic clear cell renal cell carcinoma. Ann Oncol. 2016;27(7):1304–11.
Derosa L, Rouche JA, Colomba E, Baciarello G, Routy B, Albiges L, et al. Efficacy of cabozantinib (C) after PD-1/PD-L1 checkpoint inhibitors in metastatic Renal Cell Carcinoma (mRCC): the Gustave Roussy experience. Ann Oncol. 2017;28(suppl_5):v295–329.
Auvray M, Auclin E, Barthelemy P, Bono P, Kellokumpu-Lehtinen P, Gross-Goupil M, et al. Second-line targeted therapies after nivolumab-ipilimumab failure in metastatic renal cell carcinoma. Eur J Cancer. 2019;108:33–40.
Shah AY, Kotecha RR, Lemke EA, Chandramohan A, Chaim JL, Msaouel P, et al. Outcomes of patients with metastatic clear-cell renal cell carcinoma treated with second-line VEGFR-TKI after first-line immune checkpoint inhibitors. Eur J Cancer. 2019;114:67–75.
Dudani S, Graham J, Wells JC, Bakouny Z, Pal SK, Dizman N, et al. First-line immuno-oncology combination therapies in metastatic renal-cell carcinoma: results from the international metastatic renal-cell carcinoma database consortium. Eur Urol. 2019;76(6):861–7.
Cao X, Tang D, Ratto B, Poole A, Ravichandran S, Jin L, et al. Real-world clinical outcomes of pazopanib immediately after discontinuation of immunotherapy for advanced renal cell carcinoma. Clin Genitourin Cancer. 2020;18(1):e37–45.
Navani V, Wells JC, Boyne DJ, Cheung WY, Brenner DM, McGregor BA, et al. CABOSEQ: the effectiveness of cabozantinib in patients with treatment refractory advanced renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC). Clin Genitourin Cancer. 2022;21:S1558-7673(22)00160-4.
Gan CL, Dudani S, Wells JC, Donskov F, Pal SK, Dizman N, et al. Cabozantinib real-world effectiveness in the first-through fourth-line settings for the treatment of metastatic renal cell carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Cancer Med. 2021;10(4):1212–21.
McGregor BA, Lalani AA, Xie W, Steinharter JA, Bakouny ZE, Martini DJ, et al. Activity of cabozantinib after immune checkpoint blockade in metastatic clear-cell renal cell carcinoma. Eur J Cancer. 2020;135:203–10.
Wiele AJ, Bathala TK, Hahn AW, Xiao L, Duran M, Ross JA, et al. Lenvatinib with or without everolimus in patients with metastatic renal cell carcinoma after immune checkpoint inhibitors and vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapies. Oncologist. 2021;26(6):476–82.
Graham J, Shah AY, Wells JC, Mckay RR, Vaishampayan U, Hansen A, et al. Outcomes of patients with metastatic renal cell carcinoma treated with targeted therapy after immuno-oncology checkpoint inhibitors. Eur Urol Oncol. 2021;4(1):102–11.
Powles T, Motzer RJ, Escudier B, Pal S, Kollmannsberger C, Pikiel J, et al. Outcomes based on prior therapy in the phase 3 METEOR trial of cabozantinib versus everolimus in advanced renal cell carcinoma. Br J Cancer. 2018;119(6):663–9.
Ornstein MC, Pal SK, Wood LS, Torner JM, Hobss BP, Jia XS, et al. Individualised axitinib regimen for patients with metastatic renal cell carcinoma after treatment with checkpoint inhibitors: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2019;20(10):1386–94.
Procopio G, Pircher C, Claps M, Guadalupi V, Mennitto A, Sepe P, et al. A phase II open-label study of cabozantinib after first-line treatment including an immune-checkpoint combination in patients with advanced or unresectable renal cell carcinoma: the BREAKPOINT trial (MeetUro trial 03 - EudraCT number 2018-000582-36). J Clin Oncol. 2021;39(6_suppl):326–326.
Grande E, Alonso-Gordoa T, Reig O, Esteban E, Castellano D, Garcia-del-Muro X, et al. Results from the INMUNOSUN-SOGUG trial: a prospective phase II study of sunitinib as a second-line therapy in patients with metastatic renal cell carcinoma after immune checkpoint-based combination therapy. ESMO Open. 2022;7(2): 100463 (Epub 2022 Apr 8).
Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ullbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs—part A: renal, penile, and testicular tumours. Eur Urol. 2016;70:93–105.
de Velasco G, McKay RR, Lin X, Moreira MB, Simantov R, Choueiri TK. Comprehensive analysis of survival outcomes in non–clear cell renal cell carcinoma patients treated in clinical trials. Clin Genitourin Cancer. 2017;15(6):652-660.e1.
Vera-Badillo FE, Templeton AJ, Duran I, Ocana A, de Gouveia P, Aneja P, et al. Systemic therapy for non-clear cell renal cell carcinomas: a systematic review and meta-analysis. Eur Urol. 2015;67(4):740–9.
Heng DYC, Wells JC, Rini BI, Beuselinck B, Lee J-L, Knox JJ, et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol. 2014;66(4):704–10.
Hudes G, Carducci M, Tomczak P, Dutcher J, Fligin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81.
Armstrong AJ, Halabi S, Eisen T, Broderic S, Stadler WM, Jones RJ, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncol. 2016;17:378–88.
Tannir NM, Jonasch E, Albiges L, Altinmakas E, Ng CS, Martin SF, et al. Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): a randomized multicenter phase 2 trial. Eur Urol. 2016;69:866–74.
Motzer RJ, Barrios CH, Kim TM, Falcon S, Cosgriff F, Harker WG, et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol. 2014;32:2765–72.
Fernández-Pello S, Hofmann F, Tahbaz R, Marconi L, Lam TB, Albiges L, et al. A systematic review and meta-analysis comparing the effectiveness and adverse effects of different systemic treatments for non-clear cell renal cell carcinoma. Eur Urol. 2017;71:426–36.
Park I, Lee SH, Lee JL. A multicenter phase II trial of axitinib in patients with recurrent or metastatic non-clear-cell renal cell carcinoma who had failed prior treatment with temsirolimus. Clin Genitourin Cancer. 2018;16:e997-1002.
Jung KS, Lee SJ, Park SH, Lee J-L, Lee S-H, Lim JY, et al. Pazopanib for the treatment of non-clear cell renal cell carcinoma: a single-arm, open-label, multicenter, phase II study. Cancer Res Treat. 2018;50:488–94.
Buti S, Bersanelli M, Maines F, Facchini G, Gelsomino F, Zustovich F, et al. First-line pazopanib in non-clear-cell renal carcinoma: the Italian retrospective multicenter PANORAMA study. Clin Genitourin Cancer. 2017;15:e609–14.
Campbell MT, Bilen MA, Shah AY, Lemke E, Jonasch E, Venkatesan AM, et al. Cabozantinib for the treatment of patients with metastatic non-clear cell renal cell carcinoma: a retrospective analysis. Eur J Cancer. 2018;104:188–94.
Prisciandaro M, Ratta R, Massari F, Fornarini G, Caponnetto S, Iacovelli R, et al. Safety and efficacy of cabozantinib for metastatic nonclear renal cell carcinoma: real-world data from an Italian managed access program. Am J Clin Oncol. 2019;42:42–5.
Linehan WM, Spellman PT, Ricketts CJ, Creighton CJ, Fei SS, Davis C, et al. Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med. 2016;374(2):135–45.
Suárez C, Lee J-L, Ziobro M, Gafanov RA, Matveev VB, Donskov F, et al. First-line pembrolizumab (pembro) monotherapy for advanced non-clear cell renal cell carcinoma (nccRCC): updated follow-up for KEYNOTE-427 cohort B. Ann Oncol. 2019. https://doi.org/10.1093/annonc/mdz249.044.
Vogelzang NJ, McFarlane JJ, Kochenderfer MD, Molina AM, Arrowsmith E, Bauer TM, et al. Efficacy and safety of nivolumab in patients with non-clear cell renal cell carcinoma (RCC): results from the phase IIIb/IV CheckMate 374 study. J Clin Oncol. 2019;37(7_suppl):562.
De Giorgi U, Cartení G, Giannarelli D, Basso U, Galli L, Cortesi E, et al. Safety and efficacy of nivolumab for metastatic renal cell carcinoma: real-world results from an expanded access programme. BJU Int. 2019;123(1):98–105.
McKay RR, McGregor BA, Gray K, Steinharter JA, Walsh MK, Braun DA, et al. Results of a phase II study of atezolizumab and bevacizumab in non-clear cell renal cell carcinoma (nccRCC) and clear cell renal cell carcinoma with sarcomatoid differentiation (sccRCC). J Clin Oncol. 2019;37(7_suppl):548.
Suárez Rodríguez C, Larkin J, Patel PM, Pérez-Valderrama B, Rodríguez-Vida A, Glen H, et al. Clinical activity of durvalumab and savolitinib in MET-driven, metastatic papillary renal cancer. J Clin Oncol. 2021;39(15_suppl): abstr4511.
Lee CH, Voss MH, Carlo MS, Chen YB, Zucker M, Knezevic A, et al. Nivolumab plus cabozantinib in patients with non-clear cell renal cell carcinoma: Results of a phase 2 trial. J Clin Oncol. 2021;39(suppl 15): abstr4509.
Haas NB, Lin X, Manola J, Pins M, Liu G, McDermott D, et al. A phase II trial of doxorubicin and gemcitabine in renal cell carcinoma with sarcomatoid features: eCOG 8802. Med Oncol. 2012;29(2):761–7.
Escudier B, Droz JB, Rolland F, Gravis G, Beuzeboc P, Chauvet B, et al. Doxorubicin and ifosfamide in patients with metastatic sarcomatoid renal cell Carcinoma: a phase II study of the genitourinary group of the French Federation of Cancer Centers. J Urol. 2002;168(3):959–61.
Acknowledgements
The authors thank Sergio Vázquez for the review and validation of the levels of evidence and grades of recommendation in this guideline.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MJMV reports Advisory Board—Speaker—Non-financial Support from BMS and Merck; Advisory Board—Speaker from Pfizer; Advisory Board from EISAI and MSD; Speaker from Eusa; Speaker—Non-financial Support from ROCHE; Advisory Board—Non-financial Support from ASTRA ZENECA; from Ipsen. MLQ reports Speaker from Eisai and BMS; Advisory Board—Speaker from Roche, Astellas, AstraZeneca, Sanofi and Merck; Advisory Board—Speaker—Grant from MSD and Ipsen; Advisory Board from Pfizer; Board—Grant from Bayer; Grant from Janssen; Speaker—Grant from Takeda. BPV reports Advisory Board and Speaker from BMS MSD Oncology and Astellas Pharma; Advisory Board from AstraZeneca and Novartis; Speaker from Bayer, Roche, Ipsen and Eusa Pharma; Speaker and other from Merck and Pfizer. CSR reports Advisory Board and Personal Feels from Bristol-Myers Squibb (Inst), Pfizer, Sanofi, MSD, Ipsen, Roche and Astellas; Grant from Ipsen; from Bayer. JAA reports Advisory Board and Speaker from Pfizer and Merck; Adisory Board from Astellas, MSD, AstraZeneca, Bayer, Novartis, Ipsen and Eusa; Advisory Board and Grant from BMS. IPF reports Advisory Board from Ipsem, Pfizer and MSD; Speaker from Pfizer and Ipsen; Other from Ipsen and Pfizer. EGD reports Board—Speaker—Grant—Non-financial Support from Sanofi, Janssen, Astellas, Bayer, Roche, BMS, Ipsen and Pfizer; Advisory Board—Non-financial Support from AstraZeneca; Advisory Board—Speaker from Rovi, Daiichi Sankyo, Advanced Accelerator Applications and Lilly; Advisory Board—Speaker—Non-financial Support from Eisai; Advisory Board—Speaker—Grant from Merck. JLS reports Advisory Board and Speaker from Ipsen and MSD; Speaker from BMS, Eusa and Pfizer. AGdA has received research funding from Astellas, travel grants from Astellas, Jansen, Sanofi, BMS, Roche, Pfizer, MSD, AZ and Ipsen and honoraria for speaker engagements, advisory boards and continuous medical education from Janssen, Astellas, Sanofi, Bayer, Roche, Ipsen, BMS, MSD, Pfizer, Eusa Pharma, Eisai, Novartis, AAA and AstraZeneca. NLM have nothing to disclose.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Méndez-Vidal, M.J., Lázaro Quintela, M., Lainez-Milagro, N. et al. SEOM SOGUG clinical guideline for treatment of kidney cancer (2022). Clin Transl Oncol 25, 2732–2748 (2023). https://doi.org/10.1007/s12094-023-03276-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03276-5