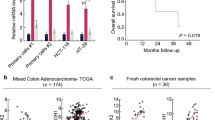
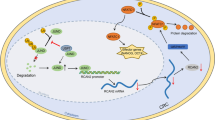
Abstract
Background
Cancer stem cells (CSCs) have unique biological characteristics, including tumorigenicity, immortality, and chemoresistance. Colorectal CSCs have been identified and isolated from colorectal cancers by various methods. AKAP12, a scaffolding protein, is considered to act as a potential suppressor in colorectal cancer, but its role in CSCs remains unknown. In this study, we investigated the function of AKAP12 in Colorectal CSCs.
Methods
Herein, Colorectal CSCs were enriched by cell culture with a serum-free medium. CSC-associated characteristics were evaluated by Flow cytometry assay and qPCR. AKAP12 gene expression was regulated by lentiviral transfection assay. The tumorigenicity of AKAP12 in vivo by constructing a tumor xenograft model. The related pathways were explored by qPCR and Western blot.
Results
The depletion of AKAP12 reduced colony formation, sphere formation, and expression of stem cell markers in colorectal cancer cells, while its knockdown decreased the volume and weight of tumor xenografts in vivo. AKAP12 expression levels also affected the expression of stemness markers associated with STAT3, potentially via regulating the expression of protein kinase C.
Conclusion
This study suggests Colorectal CSCs overexpress AKAP12 and maintain stem cell characteristics through the AKAP12/PKC/STAT3 pathway. AKAP12 may be an important therapeutic target for blocking the development of colorectal cancer in the field of cancer stem cells.







Similar content being viewed by others
Data availability
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Abbreviations
- CSCs:
-
Cancer stem cells
- CCSCs:
-
Colorectal cancer stem cells
- AKAP12:
-
A-kinase anchor protein 12
- STAT3:
-
Signal transduction and transcription activator 3
- Lv-hAKAP12:
-
Lentiviruses overexpressing AKAP12
- Lv-Null:
-
Negative control
- Lv-siAKAP12:
-
Lentiviruses with AKAP12-siRNA
- Lv-siCont:
-
Negative control
- PKC:
-
Protein kinase C
- qPCR:
-
Quantitative polymerase chain reaction
- RT-PCR:
-
Reverse transcription PCR
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. Cancer J Clin. 2021;71(1):7–33.
Marquardt S, Solanki M, Spitschak A, Vera J, Putzer BM. Emerging functional markers for cancer stem cell-based therapies: understanding signaling networks for targeting metastasis. Semin Cancer Biol. 2018;53:90–109.
Di Franco S, Bianca P, Sardina DS, Turdo A, Gaggianesi M, Veschi V, et al. Adipose stem cell niche reprograms the colorectal cancer stem cell metastatic machinery. Nat Commun. 2021;12(1):5006.
Najafi M, Farhood B, Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy. J Cell Physiol. 2019;234(6):8381–95.
Dianat-Moghadam H, Heidarifard M, Jahanban-Esfahlan R, Panahi Y, Hamishehkar H, Pouremamali F, et al. Cancer stem cells-emanated therapy resistance: Implications for liposomal drug delivery systems. J Controll Releas : Off J Controll Releas Soc. 2018;288:62–83.
Jarrett SG, Wolf Horrell EM, D’Orazio JA. Retraction: AKAP12 mediates PKA-induced phosphorylation of ATR to enhance nucleotide excision repair. Nucleic Acids Res. 2020;48(20):11814.
Wu X, Luo Y, Wang S, Li Y, Bao M, Shang Y, et al. AKAP12 ameliorates liver injury via targeting PI3K/AKT/PCSK6 pathway. Redox Biol. 2022;53: 102328.
Liu W, Guan M, Hu T, Gu X, Lu Y. Re-expression of AKAP12 inhibits progression and metastasis potential of colorectal carcinoma in vivo and in vitro. PLoS ONE. 2011;6(8): e24015.
Suren D, Yildirim M, Alikanoglu AS, Kaya V, Yildiz M, Dilli UD, et al. Lack of relation of AKAP12 with p53 and Bcl-2 in colorectal carcinoma. Asian Pac J Cancer Prev : APJCP. 2014;15(8):3415–8.
Su B, Bu Y, Engelberg D, Gelman IH. SSeCKS/Gravin/AKAP12 inhibits cancer cell invasiveness and chemotaxis by suppressing a protein kinase C- Raf/MEK/ERK pathway. J Biol Chem. 2010;285(7):4578–86.
Lee SW, Jung KH, Jeong CH, Seo JH, Yoon DK, Suh JK, et al. Inhibition of endothelial cell migration through the downregulation of MMP-9 by A-kinase anchoring protein 12. Mol Med Rep. 2011;4(1):145–9.
Yang JM, Lee HS, Seo JH, Park JH, Gelman IH, Lo EH, et al. Structural environment built by AKAP12+ colon mesenchymal cells drives M2 macrophages during inflammation recovery. Sci Rep. 2017;7:42723.
Bots M, Verbrugge I, Martin BP, Salmon JM, Ghisi M, Baker A, et al. Differentiation therapy for the treatment of t (8; 21) acute myeloid leukemia using histone deacetylase inhibitors. Blood. 2014;123(9):1341–52.
Rasanen K, Lehtinen E, Nokelainen K, Kuopio T, Hautala L, Itkonen O, et al. Interleukin-6 increases expression of serine protease inhibitor Kazal type 1 through STAT3 in colorectal adenocarcinoma. Mol Carcinog. 2016;55(12):2010–23.
Heichler C, Scheibe K, Schmied A, Geppert CI, Schmid B, Wirtz S, et al. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut. 2020;69(7):1269–82.
Venkatesan N, Wong JF, Tan KP, Chung HH, Yau YH, Cukuroglu E, et al. EZH2 promotes neoplastic transformation through VAV interaction-dependent extranuclear mechanisms. Oncogene. 2017. https://doi.org/10.1038/onc.2017.309.
Wang W, Guo C, Zhu P, Lu J, Li W, Liu C, et al. Phosphorylation of STAT3 mediates the induction of cyclooxygenase-2 by cortisol in the human amnion at parturition. Sci Signal. 2015;8(400):ra106.
Turtoi A, Mottet D, Matheus N, Dumont B, Peixoto P, Hennequiere V, et al. The angiogenesis suppressor gene AKAP12 is under the epigenetic control of HDAC7 in endothelial cells. Angiogenesis. 2012;15(4):543–54.
Mostafa MR, Yahia RS, Abd El Messih HM, El-Sisy E, El Ghannam DM. Gravin gene expression in acute myeloid leukemia. Med Oncol (Northwood, London, England). 2013;30(2):548.
Peixoto P, Blomme A, Costanza B, Ronca R, Rezzola S, Palacios AP, et al. HDAC7 inhibition resets STAT3 tumorigenic activity in human glioblastoma independently of EGFR and PTEN: new opportunities for selected targeted therapies. Oncogene. 2016;35(34):4481–94.
Jo U, Whang YM, Kim HK, Kim YH. AKAP12alpha is associated with promoter methylation in lung cancer. Cancer Res Treat : Off J Korean Cancer Assoc. 2006;38(3):144–51.
Cosenza M, Civallero M, Pozzi S, Marcheselli L, Bari A, Sacchi S. The combination of bortezomib with enzastaurin or lenalidomide enhances cytotoxicity in follicular and mantle cell lymphoma cell lines. Hematol Oncol. 2015;33(4):166–75.
Shaheen S, Ahmed M, Lorenzi F, Nateri AS. Spheroid-formation (Colonosphere) assay for in vitro assessment and expansion of stem cells in colon cancer. Stem Cell Rev. 2016;12(4):492–9.
Yang Z, He L, Lin K, Zhang Y, Deng A, Liang Y, et al. The KMT1A-GATA3-STAT3 Circuit is a novel self-renewal signaling of human bladder cancer stem cells. Clin Cancer Res : Off J Am Assoc Cancer Res. 2017. https://doi.org/10.1158/1078-0432.CCR-17-0882.
Shien K, Papadimitrakopoulou VA, Ruder D, Behrens C, Shen L, Kalhor N, et al. JAK1/STAT3 Activation through a proinflammatory cytokine pathway leads to resistance to molecularly targeted therapy in non-small cell lung cancer. Mol Cancer Ther. 2017;16(10):2234–45.
Hatano Y, Fukuda S, Hisamatsu K, Hirata A, Hara A, Tomita H. Multifaceted interpretation of colon cancer stem cells. Int J Mol Sci. 2017. https://doi.org/10.3390/ijms18071446.
Wang R, Wei J, Zhang S, Wu X, Guo J, Liu M, et al. Peroxiredoxin 2 is essential for maintaining cancer stem cell-like phenotype through activation of Hedgehog signaling pathway in colon cancer. Oncotarget. 2016;7(52):86816–28.
Najafi M, Mortezaee K, Majidpoor J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019;234: 116781.
Srivastava AK, Banerjee A, Cui T, Han C, Cai S, Liu L, et al. Inhibition of miR-328-3p impairs cancer stem cell function and prevents metastasis in ovarian cancer. Can Res. 2019;79(9):2314–26.
Wang P, Wan WW, Xiong SL, Feng H, Wu N. Cancer stem-like cells can be induced through dedifferentiation under hypoxic conditions in glioma, hepatoma and lung cancer. Cell Death Discov. 2017;3:16105.
Zeuner A, Todaro M, Stassi G, De Maria R. Colorectal cancer stem cells: from the crypt to the clinic. Cell Stem Cell. 2014;15(6):692–705.
Soheilifar MH, Moshtaghian A, Maadi H, Izadi F, Saidijam M. BMI1 Roles in cancer stem cells and its association with MicroRNAs dysregulation in cancer: emphasis on colorectal cancer. Int J Cancer Manag. 2018. https://doi.org/10.5812/ijcm.82926.
Zhu Y, Huang S, Chen S, Chen J, Wang Z, Wang Y, et al. SOX2 promotes chemoresistance, cancer stem cells properties, and epithelial-mesenchymal transition by β-catenin and Beclin1/autophagy signaling in colorectal cancer. Cell Death Dis. 2021;12(5):449.
Zhang M, Peng R, Wang H, Yang Z, Zhang H, Zhang Y, et al. Nanog mediated by FAO/ACLY signaling induces cellular dormancy in colorectal cancer cells. Cell Death Dis. 2022;13(2):159.
Yan X, Liu L, Li H, Qin H, Sun Z. Clinical significance of Fusobacterium nucleatum, epithelial-mesenchymal transition, and cancer stem cell markers in stage III/IV colorectal cancer patients. Onco Targets Ther. 2017;10:5031–46.
Liu W, Guan M, Su B, Ye C, Li J, Zhang X, et al. Quantitative assessment of AKAP12 promoter methylation in colorectal cancer using methylation-sensitive high resolution melting: correlation with Duke’s stage. Cancer Biol Ther. 2010;9(11):862–71.
Maki T, Choi YK, Miyamoto N, Shindo A, Liang AC, Ahn BJ, et al. A-kinase anchor protein 12 is required for oligodendrocyte differentiation in adult white matter. Stem Cells (Dayton, Ohio). 2018. https://doi.org/10.1002/stem.2771.
Deng Y, Gao J, Xu G, Yao Y, Sun Y, Shi Y, et al. HDAC6-dependent deacetylation of AKAP12 dictates its ubiquitination and promotes colon cancer metastasis. Cancer Lett. 2022;549: 215911.
Liu W, Kovacevic Z, Peng Z, Jin R, Wang P, Yue F, et al. The molecular effect of metastasis suppressors on Src signaling and tumorigenesis: new therapeutic targets. Oncotarget. 2015;6(34):35522–41.
Akakura S, Gelman IH. Pivotal role of AKAP12 in the regulation of cellular adhesion dynamics: control of cytoskeletal architecture, cell migration, and mitogenic signaling. J Sign Transduct. 2012;2012: 529179.
Yao C, Su L, Shan J, Zhu C, Liu L, Liu C, et al. IGF/STAT3/NANOG/Slug signaling axis simultaneously controls epithelial-mesenchymal transition and stemness maintenance in colorectal cancer. Stem Cells. 2016;34(4):820–31.
Jin W. Role of JAK/STAT3 signaling in the regulation of metastasis, the transition of cancer stem cells, and chemoresistance of cancer by epithelial-mesenchymal transition. Cells. 2020. https://doi.org/10.3390/cells9010217.
Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C, et al. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Can Res. 2011;71(23):7226–37.
Acknowledgements
All authors sincerely thank the Central Laboratory of Shanghai Tenth People's Hospital for providing experimental equipment and experimental platform for this research.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The research involving animals strictly abides by the ethical regulations of Longhua Hospital affiliated to Shanghai University of Traditional Chinese Medicine and has been approved by the ethics committee.
Consent for publication
Not applicable.
Informed consent statement
For this type of study, informed consent statement is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12094_2023_3230_MOESM1_ESM.tif
Sup. Fig. 1:The proportion of cells staying in S-phase and G2M phase was increased in HCT116-hAKAP12 cells, Supplementary file1 (TIF 1007 KB)
12094_2023_3230_MOESM2_ESM.tif
Sup. Fig. 2:The proportion of cells staying in S-phase and G2M phase was increased in LoVo-hAKAP12 cells, Supplementary file2 (TIF 124 KB)
12094_2023_3230_MOESM3_ESM.tif
Sup. Fig. 3:Statistical graph of the corresponding WB results in Figure 5. A-D correspond to Figure 5B, D, F and H respectively, Supplementary file3 (TIF 201 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, K., Wu, X., Li, Y. et al. AKAP12 promotes cancer stem cell-like phenotypes and activates STAT3 in colorectal cancer. Clin Transl Oncol 25, 3263–3276 (2023). https://doi.org/10.1007/s12094-023-03230-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03230-5